ACTs have shown great promise in cancer treatment; however, their clinical success remains challenged by several obstacles. Despite these hurdles, ongoing research and innovation are continuously advancing strategies to enhance their effectiveness and overcome existing limitations.
4.1. Challenges in ACT
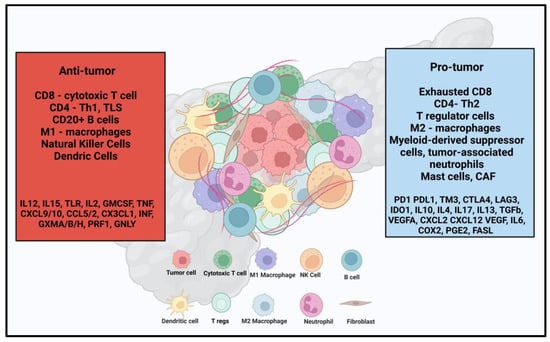
Immunosuppressive TME is an independent poor prognostic factor and presents a significant challenge to achieving effective outcomes with immune therapies, including ICI and ACT [
31]. These challenges are not unique to PDAC but impact their broader clinical application. The success of CAR-T therapy largely depends on the selection of appropriate targets. Many current studies have repurposed tumor-associated antigens (TAAs) from other malignancies—such as CEA, Claudin, mesothelin, EGFR, and HER2—with varying degrees of success. The modest responses observed in past clinical trials, combined with a high risk of toxicities such as CRS and ICANS, emphasize the need to identify more reliable target antigens. Key concerns include antigen escape (loss of target antigen expression in tumor cells), off-target effects (attack on normal cells expressing the target antigen), and the potential for secondary malignancies [
46,
113]. CAR-T cell exhaustion, characterized by impaired T-cell proliferation and effector function due to persistent antigen stimulation, remains a major cause of treatment resistance and tumor relapse [
114].
Similar challenges exist with TCR-T therapies, including target selection, exhaustion, toxicity, and off-target effects. Additionally, TCRs are restricted to recognizing antigens presented by HLA class I molecules, which may limit their applicability to tumors with heterogeneous antigen presentation [
115]. Despite the advantages of CAR-NK therapy over CAR-T therapy, challenges such as difficulty in ex vivo expansion, freezing and storage limitations, lower cytotoxicity compared to CAR-T cells, low circulating levels, and limited commercial partnerships hinder its clinical adoption [
116].
In contrast, TIL therapy offers the advantage of using autologous cells without extensive genetic modification, thereby reducing toxicity risks. However, several logistical and economic barriers hinder its widespread use, including the requirement for fresh tumor tissue, the labor-intensive process of generating and expanding TILs with IL-2, anti-CD3, and feeder cells, and the eventual reinfusion into the patient [
117]. Furthermore, conditioning chemotherapy (cyclophosphamide and fludarabine) used prior to TIL infusion often results in bone marrow suppression, while IL-2 administration is associated with adverse effects such as tachycardia, hypotension, rash, diarrhea, dyspnea, anuria, and edema [
118]. The heterogeneity of cell populations is the main drawback of CIK cellular therapies [
119]. An increased presence of CD4+ T cells and regulatory T cells (Tregs) within the TME can impair cytotoxicity and induce T-cell exhaustion, ultimately reducing the treatment’s efficacy [
120].
In conclusion, while immune therapies such as CAR-T, TCR-T, TIL, CAR-NK, and CIK therapies hold significant promise for the treatment of PDAC and other malignancies, several challenges must be addressed to optimize their efficacy and safety.
4.2. Future Directions in ACT
Enhancing the TME to reduce immunosuppression and improve T-cell infiltration is crucial for the success of ACTs. Strategies such as targeting angiogenesis, depleting tumor-associated macrophages, and inhibiting cancer-associated fibroblasts responsible for the dense extracellular matrix have shown promise in preclinical studies [
121,
122,
123,
124,
125,
126]. Therapeutic approaches involving vascular endothelial growth factor A (VEGFA) and angiopoietin-2 inhibitors, as well as other signaling pathways, are currently being explored in combination with ACTs [
127,
128,
129,
130].
Identifying novel PDAC-specific targets is crucial for advancing CAR-T and TCR therapies. Several promising targets currently under investigation for PDAC include natural killer group 2D (NKG2D), fibroblast activation protein (FAP), CD318, TSPAN8, and CD66c [
131,
132,
133]. Innovative approaches such as TCR-CAR and TCR-like CAR, which combine the strengths of CAR and TCR platforms, are being explored to enhance therapeutic efficacy [
134]. In TCR-CAR, a soluble TCR is fused to the CAR signaling tail, while TCR-like CAR incorporates TCR-like antibodies capable of recognizing the peptide/major histocompatibility complex (MHC) on tumor cell surfaces, combined with CAR signaling to improve target specificity [
135,
136]. Additionally, combining CAR-T therapy with PD-1 blockade—either via ICIs or intrinsic signaling pathway modifications—is being explored to mitigate CAR-T exhaustion and enhance efficacy [
137,
138,
139,
140,
141,
142,
143]. Early-phase studies have demonstrated potential in prostate, breast, and pleural malignancies; however, similar efforts in PDAC remain limited. Likewise, the combination of CAR-T therapy with transforming growth factor beta (TGF-β) signal blockade is being explored to counteract the immunosuppressive TME [
144,
145,
146,
147]. Additionally, CAR-T therapy is being studied in combination with other agents, including CD20-targeting antibodies (rituximab, obinutuzumab, and glofitamab), Bruton tyrosine kinase (BTK) inhibitors (acalabrutinib and ibrutinib), and lenalidomide, to further enhance efficacy [
148]. In prostate cancer, CAR-T is also being evaluated in conjunction with radiation therapy (NCT05805371), and vaccine combinations are currently under review for hematological malignancies [
148]. Encouraging similar research efforts in PDAC is critical to expanding treatment options and improving patient outcomes.
Furthermore, various novel CAR designs are being developed to prevent prolonged antigen exposure and enhance control over CAR-T cell activity, such as drug-stabilizing, drug-destabilizing, inducible (via doxycycline), self-driving (under control of AP1-NF-kB or STAT5), TME-driven (induced by IFN-γ, NFκB, and hypoxia), switch, and split CARs [
149,
150,
151,
152,
153]. The switch and split CARs are engineered by uncoupling the activation domains to prevent prolonged exposure to the target. Synthetic Notch (SynNotch) receptors and bispecific adapters, which drive the expression of CARs upon tumor antigen recognition, are in early development stages and show the potential to enhance therapeutic outcomes [
154,
155]. Synthetic TCR and antigen receptor (STAR) and T-cell receptor fusion constructs (TRuCs)-T cells integrate antigen-recognition domains to improve specificity and reduce the toxicity of TCR-T cells [
156,
157]. An antibody-based binding domain fused to TCR in TRuCs and a double-chain chimeric receptor are constructed that incorporates the antibody’s antigen-recognition domain and constant regions of TCR that engage endogenous CD3 signaling machinery in STARs.
TIL therapy continues to evolve, with combinations involving ICIs showing promise in cancers such as breast, head and neck, cervical, colon, thyroid, and melanoma [
85,
158,
159,
160,
161,
162]. Research is currently exploring next-generation TILs, including tumor-infiltrating B cells (TIL-B) and PD-1-inactivated TILs, to optimize their therapeutic efficacy, the development of modified interleukins, such as IL-2 superkines with enhanced affinity for the IL-2 β subunit and IL-15 super agonist complexes, is being investigated to enhance their clinical potential further [
163,
164,
165]. Improvement in the processes involved in TILs therapies, such as automation of various stages (cell isolation and expansion) using the latest technology, standardization of the protocols, and strong collaborations among the stakeholders (industries, hospitals, and healthcare leaders) will be crucial in scaling up TIL therapy for widespread clinical use [
166,
167].
CAR-NK cells are also being evaluated in combination with ICIs and other agents, such as lenalidomide and azacytidine, to improve their efficacy [
168]. Standardized protocols for cytokine-induced killer (CIK) cell expansion are needed to optimize cell composition (T regs and CD4 cells) and efficacy. Retrospective studies have shown promising results when combining CIK therapy with chemotherapy and ICIs, highlighting its potential in PDAC and other malignancies [
169,
170,
171,
172,
173]. Gene editing remains a critical area of research, with clustered regularly interspaced palindromic repeats-associated protein 9 (CRISPR-Cas9) technology being utilized to enhance TIL, TCR-T, and CAR-T cell function by knocking out genes such as PCDCD1 to improve persistence, reduce exhaustion, and ultimately improve the outcomes in aggressive tumors such as PDAC [
117,
174,
175,
176,
177].
In conclusion, expanding the therapeutic landscape of ACT through novel combinations and technological advancements holds promise for improving clinical outcomes. Continued research and collaboration will be critical in translating these findings into effective treatment options for PDAC and other challenging malignancies.