1. Introduction
Macrodactyly (also known as megalodactyly or digital gigantism) is a rare condition characterized by overgrowth affecting one or more fingers or toes, which is usually congenital, benign, and non-hereditary. The incidence of macrodactyly in newborns is approximately 1 in 100,000, accounting for 0.9% of upper-extremity congenital anomalies [
1]. In macrodactyly, the fingers or toes enlarge disproportionately, often asymmetrically, causing stiffness, limited mobility, discomfort, or pain, with variable proportions of bone and fat tissue overgrowth unique to each patient [
2]. This entity typically follows the growth deregulation of one or more underlying tissues (osseous, nervous, lymphatic, vascular, fibrofatty) or the abnormal accumulation of substances in one or more digits. Flatt’s classification with Upton’s modification is the most commonly used guideline [
3]. It may occur as an isolated deformity or as a part of a syndrome. Besides cosmetic concerns, surgical treatment is inevitable in patients who experience functional impairment, even though the rate of recurrence is high [
4].
This study aims to present the case of an adolescent with Proteus-like syndrome and rare cytogenetic findings and introduce a diagnostic algorithm from the pediatrician’s perspective.
2. Methods
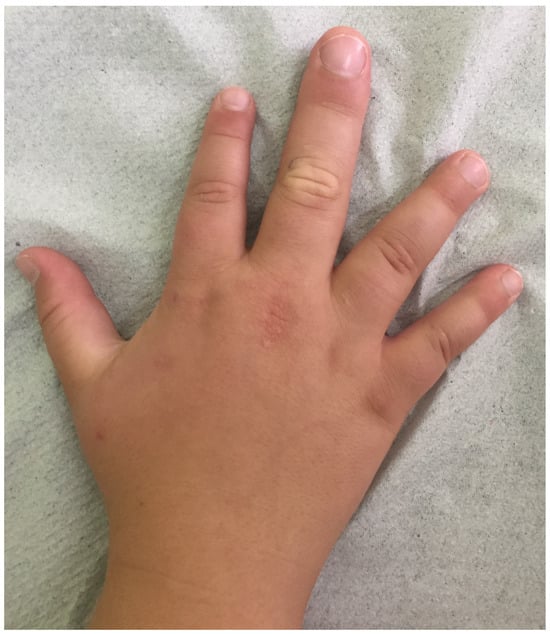
A 16-year-old Caucasian male was admitted with macrodactyly (
Figure 1) and associated functional disorders, along with dysmorphic features and autism spectrum disorder (ASD). Informed consent was obtained from his parents. Following the Declaration of Helsinki and adhering to CARE guidelines (
https://www.equator-network.org/reporting-guidelines/care/; accessed on 17 February 2025), we report that his prenatal history was unremarkable. However, his mother has bipolar disorder, migraines, and microcephaly (otherwise phenotypically normal) and gave birth to him at age 40. He was delivered full-term, with a normal birth weight and no early health concerns reported. Clinical evaluation revealed macrodactyly of the middle finger on his right hand, multiple epidermal nevi, and three lipomas on his trunk, as well as kyphoscoliosis, wide nostrils, an open mouth, and obesity (weight and body mass index at the 99th percentile for age and sex). Investigations for his heart murmur returned negative results for cardiac pathology. Regarding psychomotor development, the adolescent was found to have a low average intelligence quotient (86), with a history of slight delays in walking, significantly delayed speech, and one additional year spent in elementary school. Despite receiving special therapies for over ten years, he still exhibits some abnormal fixations. He can now talk and read simple texts or maps well but struggles with writing, numbers, and social skills. Overall, his medical history was inadequately documented, with noted gaps.
Blood tests were conducted to rule out platelet, coagulation, and endocrine disorders associated with this Proteus-like syndrome. Radiographic imaging studies assessed skeletal deformities, while abdominal ultrasonography yielded normal results. Magnetic resonance imaging (MRI) of the brain revealed no pathology. Biopsies were taken from the enlarged digit, nevus, and lipoma, and the child underwent surgery involving soft tissue debulking and physeal arrest to achieve a satisfactory functional and cosmetic outcome. Targeted analysis for pathogenic variants in AKT1 [AKT Serine/Threonine Kinase 1; c.49G>A (p.Glu17Lys)], PTEN (Phosphatase and Tensin Homolog), and PIK3CA (Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha) in the affected digit tissue returned negative results. Consequently, whole-exome sequencing (WES) of the same tissue was performed (Twist Exome Comprehensive on Illumina NovaSeq X). Given the child’s normal karyotype, we also performed comparative genomic hybridization using peripheral blood (SurePrint G3 Human Genome CGH+SNP Microarray Kit 4x180K, Agilent, Santa Clara, CA, USA).
3. Results
A 1.25 Mb copy number gain at 16p11.2-p11.1 [GRCh38/hg38 16p11.2(chr16:33836076 -35085876)x3], along with the deletion of the
GPRIN2 gene and two duplications in the region following
AKT1 at 14q32.33 (432 and 353 kb, respectively), has been reported. Corresponding studies within the family indicate that the 16p11.2 duplication was maternally inherited. WES analysis yielded results of limited significance concerning macrodactyly: one heterozygous pathogenic variant in
PRRT2 c.649delC (p.Arg217fs; located at 16p11.2) and one likely pathogenic splice variant in
SLC19A3 (c.1172+1G>A). The
PRRT2 c.649delC variant has been associated with benign familial infantile seizures and episodic kinesigenic dyskinesia, with or without infantile seizures, although there is growing evidence of incomplete penetrance [
5,
6,
7]. Given the patient’s neurological status and the lack of sufficient documentation in his medical history, it was challenging to connect this mutation with his phenotype. Certainly,
PRRT2 mutations alone cannot explain macrodactyly; therefore, the WES findings were deemed inconclusive.
In this case, macrodactyly, lipomas, and nevi raised clinical suspicion for a Proteus-like syndrome or an overgrowth syndrome affecting the PI3K-PTEN-AKT1 signaling pathway. The two copy number variants (CNVs) near
AKT1 found in our patient were not likely to be pathogenic. On the other hand, the deletion of
GPRIN2 (G-Protein-Regulated Inducer Of Neurite Outgrowth 2) could be involved in our patient’s phenotype, as it has been reported as a candidate modifier gene in different syndromes, including 16p11.2-p12.2 duplication syndrome (a genomic condition differentiated from euchromatic variations in 16p11.2), which is associated with defects in digit formation [
8,
9]. Parental psychiatric disorders have been previously associated with inherited 16p11.2 duplications. In our case, the boy’s macrodactyly led to a diagnosis for both the mother and the son. Moreover, individuals with 16p11.2 duplication are expected to display more grossly dysmorphic features compared to deletion cases (including digit malformations), but there is no recognizable pattern except microcephaly (especially in mothers) [
10]. However, no consistent pattern of physical abnormalities characterizes 16p11.2 duplications; signs and symptoms may vary even among affected members of the same family.
In summary, the genetic testing results for macrodactyly should trigger a multidisciplinary approach and detailed follow-up care. Further comprehensive studies are needed to define genotype–phenotype correlations. A diagnostic algorithm is essential in evaluating macrodactyly, particularly when syndromic or other pathological clinical features coexist.
4. Discussion
Macrodactyly is a rare condition, and surgical treatment is often insufficient unless the underlying pathology is unveiled. Most macrodactyly cases correspond to a post-zygotic somatic gain-of-function mutation in
PIK3CA in varying proportions of cells within the affected digit.
Figure 2 illustrates the PIK3K/AKT/mTOR and Ras pathways, and
Table 1 lists all entities presenting with macrodactyly according to OMIM (Online Mendelian Inheritance in Man;
https://www.omim.org/ (accessed on 17 February 2025) and relevant publications [
11,
12,
13]. The established clinical diagnostic criteria for PIK3CA-related overgrowth spectrum (PROS) require the presence of a somatic
PIK3CA mutation [
11]. There are two significant setbacks when investigating macrodactyly: (a) while macrodactyly may appear isolated, it can be part of a syndrome prior to its onset; (b) it is questionable whether genetic testing can predict outcomes and disclose follow-up actions. After reviewing the literature, a diagnostic algorithm was built for these reasons (
Figure 3).
Non-syndromic macrodactyly is more frequent than syndromic and multidigit involvement and is 2.6 times more prevalent than single-digit defects [
3]. In non-syndromic cases, our primary concerns are to investigate whether macrodactyly is progressive or static and to decipher the traits of the affected tissue. Signs of inflammation, necrosis, and suspicious radiograph findings should trigger further investigations to eliminate underlying malignancies, hamartomas (including the Sturge–Weber sequence [
12]), capillary malformations, compromised lymphatic system, and inflammatory processes. In the absence of localized symptoms and static cases, qPCR for pleiotrophin in adipose tissue can set the diagnosis, but this practice is not widespread [
14]. Contrariwise, isolated macrodactyly is usually a congenital physical anomaly that can be recognized prenatally and may require genetic counseling and testing, although there is no such consensus or guideline [
15]. Given the high prevalence of
PIK3CA mutations in cases of isolated macrodactyly, WES is strongly recommended [
1].
When syndromic features are present, and before proceeding with
PIK3CA genetic testing, we must eliminate other less common diagnoses. Macrodactyly and epiphyseal dysplasia (especially of the halluces) have been associated with tall stature and pathogenic variants in the C-type natriuretic peptide gene and its receptors [
16]. Mucopolysaccharidosis type II is a rare lysosomal storage disease that can present with macrodactyly. As it may sometimes have a mild clinical course, physicians should remain vigilant [
17]. Historically, several reports have associated neurofibromatosis with macrodactyly (primarily affecting
NF1 and, even more rarely,
NF2). Although there is no clear pathogenetic link, clinicians should eliminate this diagnosis [
3,
18]. Nevertheless, if genetic tests return negative for all the above instances, the investigations should always proceed with
PIK3CA-targeted sequencing. Of interest, the consensus for the Testing Eligibility Criteria for Somatic
PIK3CA Mutations notes that macrodactyly combined with genitourinary, gastrointestinal, and neurologic abnormalities (including autism and hypoglycemia) should prompt respective testing [
11]. Currently, research employing targeted next-generation sequencing (NGS)-based methods to explore the genetic basis of macrodactyly holds much promise [
19]. Targeted exome sequencing has recently become more accessible and has been added to clinicians’ armamentarium, while its data analysis seems to detect CNVs efficiently [
1]. At this later stage, Sanger sequencing is inferior to targeted WES in terms of time and cost-effectiveness [
20]. Based on the technical difficulties of genetic testing in these cases (mosaicism and challenges with tissue biopsy), negative WES should not be conclusive, and follow-up is warranted [
21]. In general, molecular approaches in macrodactyly investigation can include gene-targeted testing (targeted analysis, single-gene testing, multigene panel) and comprehensive genomic testing (exome sequencing, genome sequencing), depending on the phenotype. Gene-targeted testing necessitates that the clinician identifies specific genes that may be implicated, whereas genomic testing does not [
22]. The diagnostic yield of WES in neurodevelopmental disorders shows a clear benefit over CGH [
23]. While not commonly used for overgrowth syndromes, evidence supports WES as a first-tier, cost-effective approach [
24]. WES has also been used alongside RNA sequencing, but this approach is considerably too expensive [
25].
It is essential to understand the phenotypes associated with PROS because, in many cases, they are associated with high-mortality risk, while surveillance and timely surgical and/or pharmacological therapy could significantly improve the quality of life in the affected patients. An international expert consensus statement on the standard-of-care clinical practice guidelines for individuals with PROS has recently been published [
26].
Cancer incidence is not significantly increased among individuals with macrodactyly, apart from Wilms’ tumor, which is the only malignancy associated with PROS (2% of patients). Although there is insufficient evidence to demonstrate high risk, serial abdominal ultrasounds every 3–4 months until the age of 8 years are recommended in all children with a somatic
PIK3CA mutation. One case of ovarian cystadenoma has also been reported with PROS [
27].
PIK3CA mutations are prevalent in cancers that involve ectodermal or endodermal epithelia (e.g., endometrial and breast). Conversely, the affected tissue in PROS is either of mesodermal or neuroectodermal origin [
28]. Although macrodactyly is a tumorous condition, it is not considered malignant, and no malignant transformation is anticipated [
4]. However, studies of alpelisib, an alpha-specific PI3K inhibitor, in children with PROS have demonstrated efficacy and safety in alleviating symptoms [
29]. Further, macrodactyly could be diagnosed with neurofibromatosis, tuberous sclerosis, and Proteus syndrome, all of which are strongly associated with neoplasms [
12,
13,
18].
Surveillance measures in PROS include the measurement of growth parameters (including head circumference, length of arms, hands, legs, and feet) at each visit to the pediatrician, neurologic and psychomotor assessments, and a detailed examination of scoliosis and abdominal masses. Beyond the serial abdominal ultrasounds mentioned above, serial brain, spinal, and truncal MRI testing is indicated only in patients with pathologic findings in these respective regions. Consultation for endocrinopathies and hematological manifestations should be sought accordingly [
26,
30]. In our case, the success of the surgical treatment, the improvement of the patient’s psychomotor skills with therapy, the lack of new neurological or other pathological manifestations, and the genetic testing results led to follow-up with annual visits to the pediatrician. Nevertheless, the coexistence of macrodactyly with mild and nonspecific dysmorphic features, along with ASD and a history of psychomotor delay, prompted genetic testing in our case, making the prioritization of eliminating a PROS diagnosis essential. The clinical management of syndromic macrodactyly is well described by consensus guidelines. This report denotes that isolated macrodactyly also needs the attention of physicians and warrants a multidisciplinary approach as it can be, in fact, part of a syndrome. Genetic testing is not always conclusive regarding outcomes, and follow-up actions are complex to appoint.