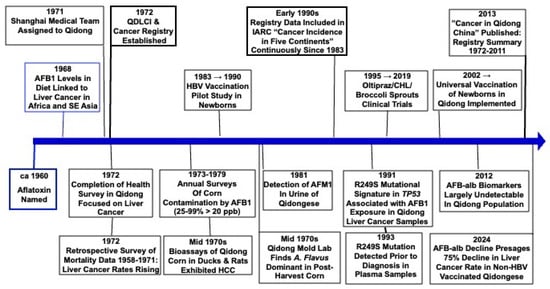
2. A Need for Aflatoxin Research in Qidong, China (1971–1992)
Research on PLC prevention and treatment in Qidong began in 1971 amidst the Cultural Revolution in China. In November 1971, the first group of the “Shanghai Tumor Research Team to Qidong County” (locally known as the “Shanghai Medical Team”) was mandated relocation from Shanghai. The team leader (1971–1973) was Dr. Lu-Yi Yu, former vice president of Shanghai Cancer Hospital. The team consisted of 13 members from various medical and research institutions, including the Shanghai Cancer Hospital, Shanghai Zhongshan Hospital, Shanghai Cancer Institute, and Shanghai First Medical College (now Fudan University Shanghai Medical College), covering disciplines such as internal medicine, gynecology, nursing, laboratory testing, and health statistics. In 1972, Jiangsu Province also sent a medical team to Qidong consisting of members from 12 scientific research and educational institutions, including clinical and preventive medicine, hydrology, soil, geology, geography, and agronomy. Among the Jiangsu Medical Team members were Dr. Yu-Tang Gao from the Department of Medical Statistics at Suzhou Medical College and Dr. Geng-Sun Qian from the Biology Department at Nanjing University (they were later transferred to the Shanghai Cancer Institute, and both later served as directors of that Institute). Their longstanding collaborative relationship with researchers in Qidong enabled international collaborations in later decades.
To detect cancer patients early, under the guidance and participation of the Shanghai and Jiangsu experts, the Qidong Health Bureau organized nearly 700 medical staff into 18 survey teams in early January 1972. Equipped with diagnostic and treatment instruments, they went deep into each village and spent nearly a year conducting a health survey focusing on liver cancer among the population aged 16 and above in Qidong County, covering 580,000 people. This survey provided an accurate understanding of the health status of the population and the prevalence of malignant tumors. In 1972, AFP (alpha-fetoprotein) testing continued, and in May and October, surveys were conducted on the population aged 16 and above and liver disease patients in the Huilong, Xiangyang, and Xining communes (townships), with a total of 71,585 people examined. Among them, 428 tested positive for AFP, and 105 cases of liver cancer were identified [
10,
11].
At the same time, additional epidemiological and etiological studies on liver cancer were initiated. These included follow-up surveys on patients with hepatitis and cirrhosis discharged from Qidong People’s Hospital since 1964, HBsAg testing, and investigations of the relationship between liver cancer and environmental factors such as water and soil, aflatoxin contamination in food, and family surveys of liver cancer patients.
To understand the trends and distribution characteristics of liver cancer incidence and mortality in Qidong, with support from the local government, broad public cooperation, and guidance from medical teams, a retrospective investigation was organized in July and August 1972. More than 750 medical personnel surveyed cancer mortality data from 1958 to 1971, covering a population of 1.03 million. The investigation involved discussions with elderly farmers, veteran party members, and senior officials, using a retrospective method of all-death causes and calculating the year of death, which helped provide a timeline for determining mortality rates. The results showed that from 1958 to 1971, the overall all-cause death rate in Qidong decreased yearly, from 8.26 to 6.62 per 1000. However, the mortality rate from malignant tumors significantly increased, rising from 56.7 per 100,000 to 124.5 per 100,000. Notably, liver cancer mortality increased substantially, from 20.5 per 100,000 to 49.0 per 100,000. Among males aged 30 to 49, for every four deaths, one was caused by liver cancer, highlighting the severe impact of liver cancer in this region. The investigation also revealed that liver cancer accounted for the highest proportion (35%) of all malignant tumor deaths, followed by stomach cancer at 29% [
11,
12]. This comprehensive survey confirmed that Qidong was a high-incidence area or hotspot for liver cancer in China.
In conjunction with the training for the retrospective mortality survey, a county-wide network for cancer control was established, with dedicated or part-time personnel assigned to engage in cancer prevention activities at all levels of medical institutions. A cancer registration and reporting system was also implemented. On 8 December 1972, the Qidong Liver Cancer Control and Research Leadership Group was established. The group was led by the vice governor of the county government, with the deputy director of the Nantong District Health Bureau, the head of the Shanghai Medical Team, and the head of the Jiangsu Medical Team as vice leaders and the director of the Qidong Health Bureau as the office director. This marked the official launch of on-site research on liver cancer prevention and treatment in Qidong and was the foundation for the Qidong Liver Cancer Institute (
Figure 2).
Multiple food collections were undertaken during the 1970s to measure the extent of aflatoxin contamination of the primary foodstuffs of Qidongese. For example, grain samples were collected in December 1972, May 1973, and September 1973, totaling 711 samples. The results indicated that corn was heavily contaminated by aflatoxin, with 95% of samples showing contamination.
Aspergillus flavus was identified in most samples; other fungi identified included
Penicillium and
Aspergillus niger [
13]. The aflatoxin B
1 (AFB
1) levels from those collections are shown in
Table 1.
A follow-up report showed that the contamination of corn with AFB
1 was substantial in Qidong from 1973 to 1980 [
14]. The contamination rate of corn ranged from a minimum of 26% to a maximum of nearly 99%, with an average of 47% (
Table 2). AFB
1 content greatly exceeded the national permissible standard (20 ppb). These findings indicate a serious problem with AFB
1 contamination in Qidong, suggesting that the region was a hotspot for aflatoxicosis, with a significantly high number of positive results across various food and grain samples. The contamination of both raw and cooked foods highlighted the serious health risk posed by AFB
1.
Isolation and culture results of mold from staple foods and grain storage containers in Qidong indicated various levels of Aspergillus flavus contamination. Corn was the most severely affected, with a contamination rate of 95%; wheat followed at 19.5%, and polished rice had the lowest at 2%. Grain storage containers, cabinets, dining tables, team warehouses, and food processing areas also showed Aspergillus flavus contamination, with an overall contamination rate of 75%.
The QDLCI isolated 4278
Aspergillus flavus strains, and 694 (~16%) were found to produce AFB
1 [
13]. This indicated that not only were grains and foods in Qidong contaminated, but the natural environment also harbored significant amounts of
Aspergillus flavus, with a considerable proportion being toxin-producing strains.
Hot and humid environments are known to foster mold growth in corn and other foodstuffs and feeds. According to the analysis of meteorological data in Qidong in the 1970s, during the corn harvest period every year (August), there was an association between the number of rainy days and the degree of toxin contamination in grain [
14].
Residents of communes with a high incidence of PLC were likely to ingest more aflatoxin from corn than those of communes with a low incidence. The contamination rate of AFB
1 in corn in the Tongxing commune with a high incidence of liver cancer was significantly higher than in the Xining commune with a low incidence of liver cancer in Qidong County. Of the positive corn samples in Tongxing, 64.5% contained more than 20 ppb of the toxin, compared with only 29.2% in Xining [
15] (
Table 3). Such “geographical pathology” provides another descriptive element of association between agent and disease.
In April 1981, 12 households were randomly selected in each of two communes of Qidong County, Hehe and Xining, and meal samples were collected for three consecutive days for the detection of AFB
1 and the calculation of the weight of staple foods consumed in 381 samples of cooked staple foods (meals) collected from the two communes. The results indicated a correlation between the detection rate of AFB
1 in cooked staple foods and the intake of the residents and the mortality (incidence) rate of liver cancer [
16] (
Table 4).
According to a survey conducted in 1974 [
12], in the communes where the mortality rate of liver cancer was more than 40/100,000, the average percentage of households with more than half of their staple foods as corn was 59.3%. In contrast, in the communes where the mortality rate of liver cancer was less than 25/100,000, the average percentage of households with more than half of their staple foods as corn was only 18.2%. The detection rate of AFB
1 in cornmeal was 38.1% in communes with a high incidence of liver cancer, whilst the detection rate in communes with a relatively low incidence of liver cancer was 9.3%. The mortality rate of liver cancer was also higher in areas with a large proportion of corn production. The detection rate of AFB
1 in corn was higher in coastal areas than inland areas, which seemed to be consistent with the geographical distribution of liver cancer.
Field Studies. In Qidong, poisoning in livestock and poultry due to feeding of moldy corn was frequently observed. This occurred particularly during the corn harvest season in August and September, when prolonged rainy weather led to increased moldy corn. Additionally, spontaneous liver cancer was commonly found in livestock and poultry.
In the Mid-Autumn Festival of 1973, 1321 duck livers were examined, and pathological tests revealed 43 cases of duck liver cancer, with an incidence rate of 3.3% [
13,
17]. According to the investigation, the feed used for the ducks, which was primarily corn, often contained aflatoxins. Over the years, rats and ducks were fed corn containing aflatoxins, and liver cancer was induced in both. Therefore, the presence of AFB
1 in Qidong’s corn was likely an important factor in the etiology of human liver cancer. The incidence of liver cancer and cirrhosis in ducks in Qidong was quite high, and the pathological changes suggested a possible link to aflatoxin poisoning. It seemed reasonable to anticipate that the same primary factor responsible for human liver cancer in Qidong was similarly involved in duck liver cancer.
Bioassays of Moldy Corn from Qidong. Several bioassays were conducted using moldy corn from Qidong. In one experiment, mallard ducks were fed corn contaminated with 90 ppb AFB
1 for 8 days, and after a 2-month interval, they were fed 30 ppb AFB
1 for a 2-year observation period. Among 75 experimental ducks, 25 developed PLC, resulting in a carcinogenic rate of 33.3%. The control group, fed uncontaminated corn, did not show any abnormalities, a difference that was statistically different [
15]. In another experiment, 36 rats were fed corn contaminated with 15–250 ppb AFB
1 for 2 years (accumulated intake of 1.17–1.32 mg AFB
1 per rat); 24 of them developed PLC, resulting in a carcinogenic rate of 66.7%. In Yangzhou, which is nearly 250 km from Qidong, ducks were fed contaminated corn from Qidong. Among 50 ducks, 22 developed various tumors, including 12 cases of PLC, resulting in a liver cancer incidence rate of 24.0% [
18]. In another study conducted in Yangzhou with moldy Qidong corn over the period of December 1973–June 1976, 80 mallard ducks, 2.5 months old and weighing about 2 kg, were divided into two groups: a moldy corn group (50 ducks) and a control group (30 ducks) [
15]. They were kept in enclosures on land, drinking local tap water, and fed a mixture of broken rice, bran, and green fodder. The moldy corn group was fed a mixture containing 150–500 ppb AFB
1, and the ducks were allowed to consume the food freely. Liver biopsies were conducted periodically, and any deaths were examined with routine histopathology. The ducks were fed moldy corn for a cumulative period of 13 months. In the first 3 months of feeding, 12 ducks died, and all were found to have acute or subacute toxic hepatitis. After 16 months of feeding, tumors began to appear, and by the end of the 29-month experiment, 22 tumors were identified. Among these, 12 were primary liver cancers (5 hepatocellular carcinoma, 4 cholangiocarcinoma, 3 mixed types). In total, 22 ducks with tumors included 1 with three different types of tumors, 9 with two types, and 12 with a single type. The male-to-female ratio for primary liver cancer was 6:6. No tumors were found in the control group, and histological examination of the liver showed no significant lesions.
Figure 3 shows a gross specimen of duck hepatocellular carcinoma and cytoarchitecture.
From November 1975 to April 1976, a total of 524 Qidongese blood samples were tested for AFB
1 [
15]. Seven samples were positive, and follow-up investigations on four of these samples revealed that the AFB
1 contamination was related to the consumption of food contaminated with AFB
1 prior to blood collection. After discontinuing the intake of AFB
1-contaminated food, follow-up blood tests showed negative results, suggesting that the presence of AFB
1 in the blood may be due to its ingestion through food.
In 1981, a thin-layer chromatographic method for detecting AFB
1 and AFM
1 in human urine was established in Qidong. In urine samples from 45 individuals who had ingested moldy corn (AFB
1 > 20 ppb). AFB
1 was detected in 2 samples (4.4%), while AFM
1 was detected in 21 samples (46.7%) [
16]. A study on the excretion levels of AFM
1 in the urine of residents from Beijing and Qidong showed that the 24 h urine AFM
1 excretion in normal individuals from Beijing was generally below 2 micrograms. In some instances, a significant increase was observed, primarily due to moldy rice consumption. In contrast, the excretion level of AFM
1 in urine from Qidong was significantly higher. Some individuals in this area had aflatoxin intake levels nearly two orders of magnitude higher than the normal population in Beijing, with an estimated annual cumulative intake exceeding 1 milligram [
19,
20].
Between 1975 and 1977, a three-year study was conducted across six townships in Qidong to track fungal infections in corn at different growth stages. Fungal infections increased as corn matured. The infection rate increased dramatically during the post-harvest dehulling and drying stages, with
Aspergillus flavus dominating the fungal population. Research suggested that adequate sunlight exposure and maintaining a moisture content below 13% could significantly reduce AFB
1 contamination during drying [
14].
Figure 4 shows Qidong Mold Lab and Medical Team members at work.
Further research into AFB
1 detection methods led to the development of a portable fluorescence detection device, which was successfully used to survey 2481 corn samples in local communities in Qidong. The device detected a contamination rate of 19.7%, and further analysis using thin-layer chromatography confirmed that 70% of the fluorescent-positive samples had AFB
1 levels exceeding 20 ppb. The use of this detection method greatly improved the efficiency of investigating and controlling aflatoxin contamination in local food sources [
14].
Additionally, anti-fungal measures to reduce fungal contamination and aflatoxin production were tested in Qidong. Early research in China showed that sodium metabisulfite was highly effective in preventing mold on wet corn, and ethylene oxide was found to be the most effective agent for preventing rice spoilage. Chemical detoxification methods, including sodium carbonate, chlorine, and lime water treatments, were tested for neutralizing AFB
1. Sodium carbonate proved to be the most effective, reducing aflatoxin levels to undetectable levels after 5 to 15 min of exposure. Chlorine treatment produced potentially harmful chlorinated proteins, limiting its practical application. Experiments on the production of starch and alcohol from aflatoxin-contaminated corn demonstrated that these processing methods could reduce AFB
1 to undetectable levels [
21]. However, although these chemical methods are more effective in decontaminating moldy corn, the possible toxic effects of adding chemicals to the food could not be ruled out, and this, together with the fact that the chemical decontamination methods affected the appearance of the grain and produced an off flavor, made them unsuitable for generalized promotion, and they were not adopted in the field. Nonetheless, this research emphasized the importance of early intervention during storage to prevent fungal contamination.
Studies on detoxification explored other various methods, including “sorting, peeling and degermination” and chemical treatments. The sorting method involved manually removing visibly contaminated corn kernels and proved effective for mildly contaminated samples and reducing AFB
1 levels. Peeling and degermination, especially wet peeling, also effectively reduced AFB
1 concentrations to below the national safety limit of 20 ppb. However, degermination resulted in significant nutritional losses, as most AFB
1 is concentrated in the germ [
21].
In conclusion, the findings from Qidong in the 1970s and early 1980s provided strong evidence that aflatoxin contamination in food sources, particularly corn, played a significant role in the high incidence of liver cancer in the region. While these studies shared the weaknesses of ecological studies in establishing causality, they underscored the necessity of increased public awareness and control measures to limit aflatoxin exposure. The correlation between animal models and human liver cancer incidence suggested that aflatoxin contamination in staple foods posed and continues to pose a severe public health risk, necessitating targeted intervention strategies in affected regions.
The fourteen-year retrospective mortality survey (1958–1971) initiated in 1972 provided valuable scientific evidence to understand the prevalence of malignant tumors in the Qidong area and to guide future research directions. This investigation not only revealed the severity of liver cancer but also underscored the growing importance of disease surveillance. A single retrospective survey reflects only past conditions, and to achieve long-term goals in cancer prevention and treatment, it was essential to establish a sustainable and effective registration and reporting system. This system would facilitate tracking of incidence trends and provide reliable data to inform national policies on cancer prevention and control.
There was a consensus on the need to establish a regular malignant tumor registration and reporting system in Qidong. Firstly, the local health prevention network in Qidong was well-established, with health centers in every township and basic medical personnel at the village level, providing robust organizational support for data collection and reporting. Additionally, China’s success in cancer registry work in Shanghai at that time served as a direct point of reference. Secondly, the experts from the Shanghai Cancer Institute (Shanghai Cancer Registry), Shanghai First Medical College, and Nanjing Medical College in epidemiology and medical statistics were also members of the “Medical Team” and played a crucial role in the establishment and operation of the Qidong Cancer Registry.
Qidong assigned a dedicated team of personnel at the village, town, district, and county levels to be responsible for case ascertainment, reporting, registration, and data management. Therefore, since the initiation of the project in 1972, the Qidong Cancer Registry has, through practical experience, adapted and optimized the methods and data classification standards from the Shanghai Cancer Registry, tailoring them to the characteristics of rural Qidong. This has resulted in the creation of a unique cancer registration and reporting system that continues to be in use today. It is the third oldest cancer registry in China and was the first established in a rural area.
In the early 1990s, Dr. Yu-Tang Gao from the Shanghai Cancer Institute (Shanghai Cancer Registry) recommended the submission of Qidong’s cancer registration data to the International Association of Cancer Registries (IACR), leading to Qidong becoming a unit member of IACR. Starting from Volume VI of the “Cancer Incidence in Five Continents” (CI5), published by the IACR and the IARC (International Agency for Research on Cancer), the cancer incidence and mortality data from Qidong for the period 1983–1987 and subsequent five-year periods have been included in the subsequent volumes of CI5 [
22,
23].
The continuous cancer registration data from Qidong for over more than 50 years has laid a solid foundation for the epidemiological, etiological, and clinical studies of liver cancer and other cancers and for the evaluation of the effectiveness of prevention measures in Qidong. As such, it has uniquely charted the impact of social, economic and environmental changes in cancer rates.
3. The Genesis of an International Collaboration Between the QDLCI and Johns Hopkins University (1993–2025)
Connections between scientists can be purposeful, serendipitous, or a combination of both. In the early 1980s, Dr. Yu-Tang Gao of the Shanghai Cancer Institute and Dr. Brian Henderson at USC were developing plans for establishing a large cancer cohort in men in Shanghai that would be linked to the Shanghai Cancer Registry. The initial focus was on lung and liver cancer. Drs. Gerald Wogan and John Groopman (a former doctoral student of Wogan) were invited to Shanghai in 1985 to discuss including their new biomarker, urinary aflatoxin-N
7-guanine, into the study design. There, Groopman met Dr. Geng-Sun Qian, leader of the Carcinogenesis Program of the Shanghai Cancer Institute. Henderson and Wogan had met in the early 1980s on seminar tours of American scientists in China, and Wogan and Qian met when the latter was on a sabbatical in 1980 at the US FDA to learn thin-layer chromatography methods for quantitation of aflatoxin contamination [
24].
A total of 18,244 men aged 45–64 years living in four small geographically distinct areas of metropolitan Shanghai were recruited into the cohort between 1986 and 1989. Participants completed questionnaires related to diet and other lifestyle factors as well as health history. By the time of a follow-up in 1990, 22 cases of PLC had been identified within the cohort and were matched to 140 controls. Analyses of biomarkers of infection with HBV (HBsAg+) and aflatoxin (AFB-N
7-guanine) showed a synergy between HBV infection and exposure to aflatoxin, together increasing the risk of liver cancer by greater than eightfold relative to HBV alone [
25,
26]. The clear message from this landmark study was that elimination of either risk factor could greatly reduce liver cancer incidence in this region. In 1993, Dr. Qian invited Drs. Groopman and Thomas Kensler (another former doctoral student of Wogan), both at Johns Hopkins University (JHU), to meet the leaders of the QDLCI to discuss collaborative ecological and interventional studies targeting liver cancer prevention. As discussed earlier, close ties between the leaders of the Shanghai Cancer Institute and the QDLCI were fostered during the work period of the “Medical Team to Qidong” in the early 1970s.
As a prelude to these discussions, two Hopkins faculty, Drs. Audrey Zarba and Lisa Jacobson, traveled to Qidong in July 1993. Accompanied by Drs. Yuan-Rong Zhu and Jian-Guo Chen, they made a site visit to several villages at Daxin (now spelled Daxing) to examine the feasibility of conducting a small, inexpensive pilot field study to measure current levels of aflatoxin exposures using a newly developed exposure biomarker, aflatoxin-albumin adducts. Thereafter, Drs. Groopman, Kensler, and Davidson, a Hopkins oncologist, traveled to Qidong to formalize the pilot study and explore longer-term opportunities for collaborations in cancer prevention (
Figure 5).
The pilot study was conducted in the township of Daxin. It comprised two waves, the first from September–December 1993 and the second from June–September 1994, post- and pre-harvest, respectively, for the annual local corn crop [
27]. From 120 consented individuals (men and women), 116 completed the first wave and provided blood samples every 2 weeks, whilst 103 completed the second wave and provided blood samples monthly. Using linear regression models, the mean aflatoxin-albumin adduct levels increased (
p < 0.05) during the 12 weeks of wave 1 and decreased (
p < 0.05) over the 4 months of wave 2. The duration of the waves was chosen to encompass several half-lives of circulating albumin (~30 days). Neither HBV surface antigen status nor gender modified either the baseline means or the temporal trends. From the 792 collected serum samples, only one sample had an undetectable level of the aflatoxin-albumin adduct. Buoyed by the consistent and high exposures to aflatoxin, enthusiasm was strong to develop interventional studies.
However, an important observation indicated that no tracking was observed. Thus, while the aflatoxin-albumin adduct demonstrated seasonal variations in population-level exposures, it provided no insight into the trajectories of individual exposure. Subsequent modeling in an aflatoxin hepatocarcinogenesis study in rats similarly indicated that a chemopreventive intervention with oltipraz dramatically reduced tumor incidence and areas under the curve for aflatoxin-albumin adduct levels but only related to protection at the population level and not the individual level [
28]. Thus, duration and frequency of sample collections needed to be considered carefully in any future prevention clinical trials.
In the early 1990s, there was an effort to identify molecular signatures, in the form of mutations, in populations with high exposures to environmental carcinogens, aflatoxin foremost among them. Mutational signatures within cancer cells provide new insights into the causes of individual cancers, fingerprinting both endogenous and exogenous factors that influence their development. Whole genome sequencing now enables the detection of thousands of mutations in many forms of cancer [
29]. The studies beginning in the 1990s used specific gene sequencing to identify a R249S mutational signature in the tumor suppressor gene
TP53 that was strongly associated with aflatoxin exposure in Qidong samples [
30,
31,
32,
33]. The mutation rate of codon 249 of
TP53 in hepatocellular carcinoma specimens collected in 1994–1997 in Qidong averaged 53.6% (52/97), which was significantly higher than that in Beijing (0/22) [
33]. Further reflecting an environmental etiology, few mutations in codon 249 have been found in liver cancer from Japan and other areas where there has been little exposure to aflatoxin [
34]. Initially, we evaluated genetic alterations in 24 liver resection specimens from Shanghai and Qidong [
32]. HBV was integrated in all patient samples. Alterations of
TP53 were present in 95% of the cases. All seven liver cancers from Qidong and three out of five from Shanghai had the aflatoxin-associated point mutation with a G to T transversion at codon 249 (R249S). Similarly, Szymañska et al. [
35] detected R249S mutations in 11 out of 16 (64%) liver cancers from Qidong. In a follow-up study utilizing serial samples from a longitudinal collection of plasma samples from a cohort of 1638 high-risk individuals in Qidong, we compared results from plasma DNA and DNA sequencing for specific mutations in 25 liver tumors [
36]. Mutations were detected in 10 samples. Analysis of 20 additional plasma-tumor pairs showed that 11 tumors and 6 plasma samples contained the specific mutation. Ten plasma samples from healthy individuals were all negative. Jackson et al. [
37] further explored the temporality of detection of this mutation in plasma before and after clinical diagnosis of liver cancer in the same patient. Sixteen patients diagnosed with liver cancer between 1997 and 2001, with plasma samples collected before and after liver cancer diagnosis, were selected for the study. In samples collected prior to liver cancer diagnosis, 22% of the plasma samples had detectable levels of the codon 249 mutation. The codon 249 mutation was detected in 45% of all plasma samples following diagnosis of cancer. Further, persistence of this mutation in plasma once it became measurable was statistically significant in repetitive samples following diagnosis. Nearly one half of the patients were positive for this marker at least 1 year, and in one case 5 years, prior to diagnosis. Huang et al. [
38] reported that the R249S mutation in
TP53 was detected at a much higher frequency in the plasma of liver cancer patients from Qidong than in specimens from cirrhotic or healthy controls also residing in Qidong. Subsequently, many studies on liver cancer mutations in populations exposed to high levels of dietary aflatoxin have found high frequencies of G-C to T-A transversions, with clustering at codon 249. The mutational signature “24” is now linked to this aflatoxin specific mutation in hepatocellular carcinoma [
39].
Whole genome sequencing in recent years has provided new insights into the contribution of mutagenic exposures to liver cancer development. Zhang et al. [
40] sequenced 49 liver tumors collected between 1990 and 2016 in Qidong to refine genetic features associated with aflatoxin exposure. The dominant pattern was G>T transversions. The
TP53 mutation frequency was very high (81.6%), and the major genotype was the R249S hotspot mutation. Additional genes harboring mutations included
TERT,
AXIN1,
CTNNB1, and
ADGRB1. In this study, the aflatoxin mutational signature, highly represented in the Qidong samples, was observed at low frequencies in tumors from the US (3.5%) and France (1.7%).
In addition, non-target genes (e.g., hypoxanthine guanine phosphoribosyltransferase gene (
HPRT)) and effector genes (e.g.,
HBV viral genome) have been interrogated. In an early study, we determined somatic mutation frequencies in human
HPRT [
41]. Ninety healthy subjects from Daxin were assigned as having low or high exposure to AFB
1, according to a dichotomization of their levels of aflatoxin-albumin adducts around the population mean.
HPRT mutant frequency was determined in individuals by a T cell clonal assay. An odds ratio (OR) of 19.3 (95%CI: 2.0~183) was demonstrated for a high
HPRT mutation frequency in individuals with high aflatoxin exposure compared with those with low aflatoxin exposure. Several mutations in the
HBV genome have been identified as well in samples from Qidong and elsewhere [
42,
43,
44,
45]. Some may hinder immune recognition and clearance of the virus from the host [
46]. However, there is little evidence linking the mutations directly to aflatoxin exposure; rather, they likely reflect the consequences of endogenous mutagenic processes.
In the decade of the 1990s, the downward inflection in liver rates was unrecognizable, and strategies for eliminating aflatoxin from the contaminated corn were elusive. Chemoprevention using agents known to enhance the detoxication of aflatoxins in vivo, reduce the burden of DNA damage to the liver, and evoke remarkable efficacy towards preventing liver cancer in animal models offered a potential means for risk reduction in this and other highly exposed populations. A series of clinical trials with a repurposed drug, an over-the-counter medicine, and a widely consumed food ensued. The development of clinical trial protocols for chemoprevention studies in Qidong required considerable reconciliation of Western and Eastern understanding of differences in cultures, trial designs, and governmental regulations (IRBs, INDs, import and export of supplies, agents, and biospecimens). First and foremost, we would utilize a synchronous timeline such that all participants would experience each milestone on the same day. This approach would require a large team to enact it—one not achievable in the West. Participants would also be recruited from a constrained geographical area, typically a single township, for logistical ease and to preserve the notion that all participants shared common features of environmental exposures, such as to aflatoxins.
Most days, these trials ran smoothly in this format, but not all days: typhoons, floods, power failures, and social-political events (chatter amongst neighbor participants; the accidental bombing of the Chinese embassy in Belgrade by NATO forces) created challenging but surmountable obstacles.
Oltipraz. The strategy the team identified for the initial clinical disruption of aflatoxin carcinogenesis in Qidong was to attempt to enhance the detoxication of aflatoxin in exposed individuals. Oltipraz, a substituted 1-2-dithiole-3-thione, was originally developed by the pharmaceutical industry as a possible treatment for schistosomiasis and was extensively evaluated in clinical trials in the early 1980s. While studying mechanisms of antischistosomiasis by oltipraz, Bueding and colleagues at Hopkins initially noted that giving the drug to mice markedly elevated glutathione levels in many tissues [
47] as well as enzymes important to carcinogen detoxication in multiple tissues [
48,
49]. These results prompted Bueding to predict that oltipraz might have cancer chemopreventive properties. A lifetime bioassay testing the efficacy of oltipraz against aflatoxin hepatocarcinogenesis in male F344 rats provided a strong justification for its use in Qidong [
50]. Additionally, in this study, oltipraz co-treatment led to a 67% reduction in the urinary elimination of aflatoxin N
7-guanine during the dosing period, indicating that protection afforded by oltipraz presumably results from the marked decrease in levels of hepatic DNA adducts. Extensive preclinical evaluation by the National Cancer Institute (NCI) showed that oltipraz was an effective anticarcinogen in nearly a score of animal models [
51].
Supported by the US NCI, a Phase IIa chemoprevention trial of oltipraz was conducted in Daxin township, Qidong, in the summer of 1995 [
52,
53]. This was a placebo-controlled, double-blind study in which 234 participants were randomized to receive placebo or 125 mg oltipraz daily or 500 mg oltipraz weekly for 8 weeks. Aflatoxin biomarkers [
54] and metabolites [
55] were used as the primary, albeit intermediary study endpoints. Compliance amongst the study participants was very good [
53]. Urinary AFM
1 levels were reduced by 51% compared with the placebo group in persons receiving the 500 mg weekly dose. No significant differences were seen in urinary AFM
1 levels in the 125 mg group compared with the placebo. This effect was thought to be due to inhibition of cytochrome P450 1A2 activity by the higher dose only. Median levels of aflatoxin-mercapturic acid (a glutathione conjugate derivative) were elevated sixfold in the 125 mg group but were unchanged in the 500 mg group. Increased aflatoxin-mercapturic acid reflects induction of aflatoxin conjugation through the actions of glutathione transferases. The apparent lack of induction in the 500 mg group probably reflects masking caused by diminished aflatoxin-8-9-epoxide formation for conjugation through the inhibition of CYP1A2 seen in this group [
56]. (A full discussion of aflatoxin metabolism is provided in a companion article in this special issue [
55].) Aflatoxin albumin adducts were measured in serum samples collected weekly. Individuals receiving 500 mg weekly but not 125 mg daily for 8 weeks showed a triphasic response to oltipraz. No effect was observed during the first month of the intervention, whereas a significant (
p = 0.001) diminution in adduct levels was observed during the second month of active intervention and during the first month of follow-up. A partial rebound in adduct levels toward baseline values was observed during the second month postintervention. Linear regression models up to week 13 confirmed a significant (
p = 0.008) weekly decline of biomarker levels in the group receiving 500 mg of oltipraz once a week [
57]. This initial study demonstrated for the first time that aflatoxin biomarkers could be modulated in humans.
Fifty-one participants (21.8%) reported adverse clinical events [
53]. An extremity syndrome, developing soon after the start of treatment, was the only event that occurred more frequently (
p = 0.002) among the active groups (18.4 and 14.1% of the daily 125 and weekly 500 mg arms, respectively) compared with the placebo (2.5%). The oltipraz arms did not differ in symptom type or severity. Further clinical evaluation of oltipraz ended several years later when the drug went off patent and synthesis of more drug proved to be too expensive relative to other agents in the NCI pipeline.
The success of this trial was a direct result of the seamless integration of the complementary expertise of the QDLCI and JHU team members. Beyond fulfilling binational regulatory requirements, delivery of clinical trial supplies, including oltipraz tablets formulated by the NCI, and recruitment of study participants in Daxin for screening and possible eligibility were key first steps (
Figure 6). Screening included medical histories, blood and urine chemistry, β-scans, EKGs, and physical exams. Because of the synchronous design of the trial, selected participants were provided with a study calendar to highlight days for taking the study drug, providing blood and urine samples, and follow-up clinic visits (
Figure 7). Overnight (12 h) urine samples were collected at participants’ homes and quickly processed at a local “urinarium” for subsequent clinical chemistry and biomarker analyses (
Figure 8).
Chlorophyllin. Chlorophyllins are semi-synthetic metal coordination complexes derived from chlorophyll. Magnesium as the metal coordination element is replaced, and the phytyl chains are removed to create water-soluble salts. A sodium-copper complex (CHL) has long been used as an over-the-counter drug for odor control in ostomy patients and to promote wound healing. CHL is also used as a food colorant. Drs. Bailey and Dashwood and colleagues at Oregon State University defined the actions of CHL as a cancer chemopreventive agent. In animal models of AFB
1-induced liver cancer, administration of CHL at the same time as dietary AFB
1 exposure significantly reduced AFB
1-induced DNA damage in the livers of rainbow trout and rats [
58,
59] and dose-dependently inhibited the development of liver cancer in trout [
60]. Although the primary mode of action is thought to be the sequestration of aflatoxin by chlorophyllin in the gastrointestinal tract [
61,
62], thereby impeding absorption, we have characterized enzyme-inducing properties, as seen with oltipraz, that may also contribute to its mechanism of action [
63]. The OSU investigators conducted an unblinded crossover study of the possible effects of CHL on AFB
1 pharmacokinetic parameters among a small number of human volunteers [
64]. Fasting subjects received an Institutional Review Board-approved dose of
14C-AFB
1 (30 ng, 5 nCi) by capsule with 100 mL water, followed by normal eating and drinking after 2 h. Blood and cumulative urine samples were collected over 72 h, and
14C- AFB
1 equivalents were determined by accelerator mass spectrometry. The study revealed rapid human AFB
1 uptake and urinary elimination kinetics. CHL treatment significantly impeded AFB
1 absorption and reduced C
max and AUCs (plasma and urine) in the subjects, suggesting that CHL co-consumption may limit the bioavailability of ingested aflatoxin in humans, as they do in animal models. More broadly, this study established that microdosing studies with carcinogens have the potential to provide important insights into chemopreventive interventions and to enhance the overall clinical development and safety evaluation of preventive agents [
65].
In a randomized, double-blind, placebo-controlled chemoprevention trial conducted in other Daxin villages in 1997, CHL was determined to alter the disposition of aflatoxin [
66]. One hundred and eighty healthy adults were randomly assigned to ingest 100 mg of CHL (provided as Derafil
®) or a placebo three times a day prior to each meal for 4 months. The primary endpoint was modulation of levels of urinary aflatoxin N
7-guanine adducts collected three months into the intervention. Adherence to the study protocol was outstanding, and no adverse events were reported. However, both serum and feces were noted to be tinted green [
67]. Aflatoxin N
7-guanine was detected in 105 of 169 urine samples available from this timepoint. CHL consumption at each meal led to an overall 55% reduction (
p = 0.036) in median urinary levels of this aflatoxin DNA damage biomarker compared with the levels in those subjects taking the placebo [
66].
While proof of principle for the interceptor strategy to dampen aflatoxin bioavailability with CHL was clearly established, the practical implementation of population-scale interventions requiring thrice-daily administration potentially for decades precluded further development of this approach. While no concerns about toxicities from chronic administration of CHL have been reported, the potential for collateral increased uptake of copper or diminished uptake of some nutrients would require investigation.
Broccoli Sprouts (Sulforaphane). Although the initial oltipraz clinical trial demonstrated the proof of principle for inducing pathways leading to enhanced aflatoxin detoxication in humans, the practicality of using a drug-based method for prevention in the economically developing world is limited; instead, approaches for “frugal medicine” are needed [
68]. Many foods have high levels of enzyme inducers [
69] that could potentially enhance the detoxication of AFB
1. In the early 1990s, Dr. Paul Talalay and colleagues at JHU isolated and characterized sulforaphane from broccoli as the most potent naturally occurring inducer found anywhere in the plant kingdom [
70]. In the plant, sulforaphane is formed from the precursor glucoraphanin following its hydrolysis by the plant enzyme myrosinase. This group subsequently demonstrated that sulforaphane was an effective chemopreventive agent in animals [
71,
72]. They further characterized the pharmacokinetics of sulforaphane administered as a broccoli homogenate in human volunteers [
73,
74].
Importantly, they recognized that young plants, as opposed to mature market stage broccoli, held the highest concentration of glucoraphanin by weight [
75]. Partnering with Drs. Talalay and Fahey, we developed the first Phase II trial using broccoli sprouts. To provide a consistent dose, a beverage formed from hot water extract of 3-day-old broccoli sprouts, containing defined concentrations of glucosinolates as a stable precursor of the anticarcinogen sulforaphane, was evaluated for its ability to alter the disposition of aflatoxin in a clinical trial conducted in HeZuo, Qidong, in 2003. Twenty kilograms of broccoli seeds (
Brassica oleracea L., Italica Group) were imported, requiring a long paper trail of chops and approvals. A temporary sprouting facility was built in a QDLCI lab, and over 100 L each of glucoraphanin-rich (GRR) and placebo beverages were prepared, tittered for glucoraphanin levels, aliquoted, and frozen prior to daily administration to study participants (
Figure 9).
Two hundred healthy adults drank beverages containing either 400 or <3 μmol glucoraphanin nightly for 2 weeks. At the end of the study, urinary levels of aflatoxin N
7-guanine were slightly lower in the GRR intervention arms. However, measurement of urinary levels of sulforaphane metabolites indicated striking inter-individual differences in bioavailability. This outcome likely reflected individual differences in the rates of hydrolysis of glucoraphanin to sulforaphane by the intestinal microflora of the study participants (the plant myrosinase was destroyed when the sprouts were boiled in water to prepare the GRR beverage). Accounting for this variability, a significant inverse association was observed for excretion of total sulforaphane metabolites and aflatoxin N
7-guanine adducts in individuals receiving broccoli sprout glucosinolates [
76]. Those individuals exhibiting good bioavailability of sulforaphane had lower levels of aflatoxin biomarkers. This preliminary study illustrated the potential use of an inexpensive, easily implemented, food-based method for prevention. However, despite improvements in the rigor and sensitivity of the analytical methods used to measure the aflatoxin biomarker, it became apparent that aflatoxin exposures were dropping—substantially. The proportion of undetectable samples was increasing.
This study spawned a series of follow-up broccoli sprout-based clinical trials in Qidong and globally in a variety of health maintenance and disease prevention settings. As of 2024, well over 100 broccoli-based clinical trials are listed in
ClinicalTrials.gov. Several subsequent trials with broccoli sprout preparations were conducted in Qidong from 2009 to 2019 with an objective of improving the extent and consistency of the bioavailablity of sulforaphane as well as the organoleptic properties—principally taste—of the administered beverages [
75,
76,
77,
78,
79,
80,
81]. Because dietary exposures to aflatoxins had been dropping in the 1990s–2000s, it was no longer feasible, nor warranted (
infra), to use aflatoxin biomarkers as study endpoints. Over the past 20 years, the primary environmental concern in the region had become air pollution. A summary of the randomized clinical trials in Qidong employing aflatoxin biomarkers is provided in
Table 5The chemoprevention trials conducted in Qidong clearly established proof of principle for the strategy of enhancing aflatoxin elimination from humans. While there remains great promise for the use of chemoprevention and interception in some high-risk cohorts [
84], perhaps application to settings of heterogeneous exposures to a dietary carcinogen lacks sufficient precision for defining the target cohort. Other prevention modalities may have better cost-benefit and risk-benefit profiles.
As the third oldest cancer registry in China and the first established in a rural area, this registry has uniquely tracked the effects of social, economic, and environmental changes on cancer rates over the past 50+ years. Originally paper-based (
Figure 10), the registry is now digital, allowing for comprehensive longitudinal analyses of cancer rates [
11]. The magnitude of some changes is remarkable, as with liver and lung cancers (
Figure 11), and provides powerful insights into underlying etiologies [
84,
85,
86].
The age-standardized incidence of liver cancer in Qidong has dropped remarkably since the time of the founding of the cancer registry in 1972: from >80 per 100,000 to ~20 in men by 2022 and from 22 to 6 in women (
Figure 11). Historically, the major risk factors for liver cancer in Qidong have been infection with HBV (but not HCV), dietary exposures to aflatoxins, and the use of surface water for drinking [
18,
87]. Strategies, intended and serendipitous, have been directed at these three factors. A pilot program evaluating the safety and efficacy of a vaccine to prevent infection in newborns was undertaken in some Qidong townships from 1983–1990 [
88]. Close to universal vaccination of newborns did not occur in China until the early 2000s. Consequently, most Qidong residents over the age of 40 have not been vaccinated against HBV. In 2021, the median age of diagnosis of liver cancer was 67 years (IQR: 57–74). Thus, the ~75% drop in liver cancer incidence has been driven by individuals never vaccinated against HBV. Infection rates have dropped dramatically in the birth cohorts that have been vaccinated, so that there is much anticipation for the impact of the vaccination program to be seen in the upcoming decades.
It has been proposed that the initial drop in age-standardized incidence rate from the 1970s into the 1980s reflects the success of a public health initiative to replace surface pond-ditch water with deep-well water as the primary source for all Qidong residents [
87]. Deep-well water has been shown to have substantially lower levels of contamination by the blue-green algae toxin, microcystin. Microcystin is known to act as a hepatic tumor promoter in rodent models of carcinogenesis.
The increasing age of diagnosis of PLC in Qidong argues against advantages gained by improved healthcare in the region and benefits from screening programs. Indeed, survival rates following diagnosis have changed little over the past decades [
11].
By 2010, the signals from the Qidong Cancer Registry were quite strong for a robust and accelerating decline in age-standardized incidence of liver cancer. This observation prompted the QDLCI-JHU team to conduct a largely retrospective analysis of levels of aflatoxin albumin adducts in serum from samples archived from community screening programs and clinical trial eligibility screening [
86]. As depicted in
Figure 12, the percent of individuals with detectable levels of this aflatoxin exposure biomarker dropped from nearly 100% in 1995 to nearly undetectable in all samples collected in Daxin in 2012. All samples across the depicted series were age- and gender-matched. Over this 17-year period (1995–2012) the age-standardized incidence rate of liver cancer dropped from 44 per 100,000 in 1995 to 26 in 2012 when both genders are combined.
The driver for the dramatic drop in exposure to dietary aflatoxins was not through a directed public health initiative. Rather, it was the serendipitous result of economic reforms initiated by Deng Xiaoping and the Central Government in the late 1970s. Upon implementation in the mid-1980s, Qidong farmers could grow crops not just to meet local commune production quotas, but also to be sold in a broader marketplace for profit. Corn remained the primary crop in the region because of agricultural conditions unsuitable for rice cultivation, but rice became a common import from northern Jiangsu Province, whilst corn continued to be produced for use as animal feed and shipped out as an industrial raw material. Rice is far less susceptible to aflatoxin contamination than is corn (
Table 1). By 1998, only 9% of families ate any corn, whilst the proportion of rural residents consuming some rice reached >99% around this period. Further, dietary diversity featuring many fruits, vegetables, and meats arrived with economic growth in the late 1990s and likely contributed to further reductions in aflatoxin exposures [
89]. A market-driven eradication of aflatoxin from the diet was the key.