1. Introduction
Nasal steroids (NS) are very commonly prescribed in ear, nose, and throat clinics. They are preferred before and after surgery in chronic rhinosinusitis (CRS), allergic rhinitis (AR), and turbinate hypertrophies. It is observed that the use of NS is increasing due to the prevalence of AR in the world being 10–20% [
1] and the prevalence of chronic rhinosinusitis being 11% [
2]. Commonly, NS toxic effects occur with the unconscious use or overdose of nasal steroids. Different studies have revealed that the resulting damage causes a delay in wound healing, epistaxis, nasal mucosal atrophy, septal perforation, cell stress rupture, deterioration in vascular structures, and apoptosis [
3,
4]. Therefore, in order not to limit the use of NS but to reduce toxicity, in our study, the effects on the toxicity model of using propolis (from the Anatolia region) and Cur were investigated. The main purpose of our study is to use AP and Cur to eliminate the negative effects and, thus, minimize these side effects in our work with systems that allow long-term use.
Especially in nasal epithelial wound healing; fibroblast growth factor 2 (FGF-2) is involved in cell proliferation, vascular endothelial growth factor (VEGF) is involved in the formation of new vascular lines, and interleukin-25 (IL-25) and matrix metalloproteinase (MMP) are important in reducing the inflammatory process [
5,
6,
7]. VEGF and FGF-2 are powerful angiogenetic growth factors in wound healing. With the release of angiogenic substances, important processes such as the activation, adhesion, and migration of endothelial cells begin via MMP-2 [
8,
9]. Additionally, it has been shown in different studies that IL-25 contributes to the wound-healing process by supporting fibroblast activation [
10,
11,
12].
Propolis is a resinous honeybee product obtained from beehives as raw material [
13]. Propolis shows changes in chemical composition when comparing samples taken from different places and even from the same place, depending on its botanical origin. Despite chemical differences, it is well known that samples of different geographical origins and chemical compositions generally show similar biological activity. The main components of propolis are wax, resin, and volatile substances. However, the biological activity of propolis is attributed to plant-derived substances. Propolis is a protective agent against microorganisms in the hive.
In studies conducted with propolis in various nasal wounds or wounds caused by different reasons, it was found that it significantly increased and healed VEGF and FGF-2 levels [
4,
14,
15,
16]. AP contains a higher amount of phenol compounds than other types of propolis [
17], and it is known that this substance is effective in wound healing by preventing oxidative damage and nitrosative stress in the human body [
18,
19] and increasing the release of various growth hormones [
20,
21,
22].
Curcumin is a plant extract from the ginger family. Cur has an anti-inflammatory and antioxidant effect by suppressing the expression of tumor necrosis factor-alpha (TNF-α) and reactive oxygen species (ROS) [
23]. These effects lead to an increase in remodeling and the proliferative process, increasing wound healing [
24,
25,
26]. In addition, it has been shown that Cur significantly increases wound healing in nasal mucosal damage in rats, and this is due to increased angiogenesis with the release of MMP-2 and VEGF [
27,
28,
29].
Our study will demonstrate, for the first time, using AP and Cur, pure and combined, to improve the toxic effects of NS on VEGF, FGF-2, MMP-2, and IL-25 pathways. Honeybees produce AP. Propolis collected by honeybees by visiting different plants shows a wide range of biological activities (antioxidant effects). Curcumin is obtained from the turmeric plant and has been reported to have high antioxidant effects. The combined use of AP and Cur can have synergistic anti-inflammatory and antioxidant effects on the toxicity caused by BMC. To investigate this situation, two different dose-combination groups were investigated in the study. These four different pathways will be examined after 24 h using the in vitro nasal fibroblast wound model created in this way.
3. Discussion
Corticosteroids form the basis of the treatment of asthma and allergies. Corticosteroids, as regulators of the immune system, suppress the apoptosis of eosinophils and lymphocytes and the production of inflammatory eicosanoids and cytokines. However, unconscious use of corticosteroids has been shown in different studies to cause tissue damage. BCM is regularly and widely used in ear, nose, and throat diseases. Although BCM causes less toxicity than other corticosteroids, studies have shown that its toxicity causes a decrease in cell viability, oxidative stress damage, a decrease in vascularization, a decrease in cell proliferation, and an increase in inflammation when used in high doses [
3,
4,
30,
31].
A BCM IC
50 dose of 11.90 µM/mL was used. In an in vitro study, it was shown that the BCM IC50 dose inhibited the viability of fibroblasts [
32]. In our study, we observed that the viability (MTT) of nasal fibroblast cells decreased by 35% after the use of BCM (11.90 µM/mL) and this rate was statistically significant (
p < 0.01) (
Figure 1). Corticosteroids change protein synthesis in target tissues. They interact with corticosteroid receptors on the cell membrane to form a steroid-receptor complex. This complex creates a difference in second messenger stimulation and DNA expression [
33]. The study showed that Cur increased cell viability in fibroblasts [
34]. An increase in the cell viability ratio of intestinal epithelial cells was shown after Cur (5 µg/mL) administration [
35]. In addition, Cur (0.1–20 μM doses) treatment for 48 h caused an increase in the cell viability ratio (
p < 0.01) [
31]. In our study, different doses of Cur (8, 16, and 32 µM/mL) were applied to nasal fibroblast cells after treatment with BCM. It was determined that cell viability increased significantly compared to the BCM group. Similarly, there is evidence that propolis species increase cell viability in fibroblasts [
36,
37]. It was observed that AP (5 μg/mL) increased cell viability in nasal epithelial cells, although it was not statistically significant [
4]. In our study, it was observed that cell viability increased significantly (
p < 0.05) with the use of AP (2 and 2.5 mg/mL). In addition, a synergy effect was seen in the combination of Ap and Cur mainly due to antimicrobial activities, antioxidant, and anti-inflammatory effects [
38].
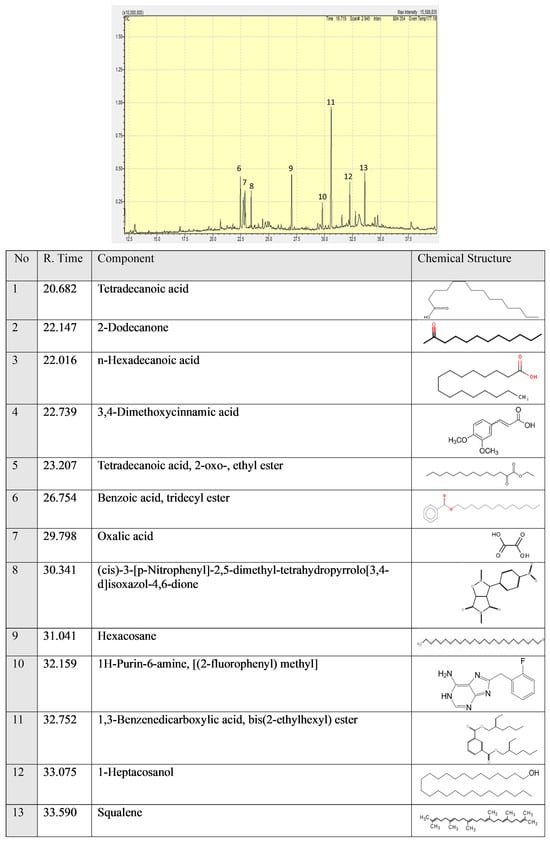
Squalene has anti-inflammation and wound-healing effects. Squalene is responsible for AP protective effects. We observed a statistically significant (
p < 0.05) increase in MTT values using AP (2 and 2.5 mg/mL). Cinnamic acid derivatives 3,4-Dimethoxycinnamic acid have antiproliferative activity against tumors [
39]. 1-heptacosanol has been reported to have antimicrobial and antioxidant activity [
40] and antibacterial activity [
41].
The maintenance of cell viability is directly related to the balance between antioxidant capacity and oxidative damage in the cell [
42]. The oxidant and antioxidant levels were determined using TAC and TOS analyses. Glucocorticoids have been shown to increase oxidative damage in cells [
43,
44]. However, there are also publications showing that Cur and Propolis cause significant changes in the values of many oxidative stress markers by reducing oxidative damage in the cell [
45,
46]. In an in vitro study by Waly MI et al., Cur (1 mM) brought the TAC values of kidney cells exposed to cisplatin up to the level of the control group [
47]. In our study, although Cur (16 mM) did not increase the TAC level as much as the control group, there was a statistically significant (
p < 0.05) difference.
Many previous studies have shown that propolis increases the TAC value and decreases the TOS value. However, there are no published studies in the literature on AP’s oxidative stress damage markers in the cell. In our study, we showed that AP (2.5 mg/mL) reduced the TAC level at a statistically significant rate (
p < 0.05), and when AP (2 mg/mL) was used, it decreased the TOS level at a statistically significant rate (
p < 0.01). A study tried to evaluate the antifibrotic effect of curcumin, N-acetyl cysteine, and propolis extract against bisphenol A-induced hepatotoxicity in rats. They showed that the antioxidant and anti-inflammatory effects of these three ingredients protect the liver from induced toxicity [
48]. In addition, we obtain better results in the in vitro study due to Anatolian propolis.
Li X et al., [
49] in their in vitro study, showed that the increased LDH level in the cell exposed to oxygen-glucose deprivation/reoxygenation was reduced to a statistically significant extent (
p < 0.01) by using Cur 5 and 10 μM. It has also been shown to increase glutathione levels significantly (
p < 0.01). In another study, Cur’s ability to reduce the toxic effects of herbicides was evaluated. It has been revealed that there is a positive correlation between Cur and GR levels [
36]. In our study, combination groups more effectively decreased LDH levels and increased GR levels. In addition, all doses of Cur act not effectively but the same as combination groups. The decrease in the LDH level was statistically significant when Cur was used at a dose of 16 μM.
The study showed that propolis can reduce oxidative stress and inflammation in rheumatoid arthritis disease in women. The results of a clinical trial showed that a reduction in oxidative stress and cytokines improved symptoms [
50]. A study by Joanna Kocot et al. showed that propolis contains many plant-derived flavonoid and phenolic components [
51]. Bees visit many different flowers because the components formulate propolis antioxidative stress and antioxidant capacity more strongly than many natural products. According to our results, the propolis effects on LDH, TAC, TOS, and Gr results are more effective than those of Cur.
Inflammation occurring in fibroblast cells leads to changes in the bronchial epithelium and lung microenvironment [
52]. Various inflammatory and pro-inflammatory markers such as transforming growth factor-beta (TGF-β), interleukin 6 (IL-6), interleukin 10 (IL-10), and interleukin 25 (IL-25) activate signal transduction and transcription activator [
44,
45]. Subsequent STAT3 phosphorylation plays an important role in the regulation of angiogenic mediators such as VEGF, FGF-2, and HIF1, leading to angiogenesis [
53]. In light of the findings from our study, it was determined that BCM caused significant differences in VEGF and FGF-2 gene levels. We think that this effect is especially effective through the STAT-3 pathway and drags the cells into inflammation and the apoptotic path. In addition, reactive oxygen species and the PI3K/AKT signaling pathway are also responsible for the expression of FGF-2. BCM-induced toxicity activated the redox system of the cells, and the apoptotic process was activated with the increase in oxidative damage. To eliminate BCM-induced damage, we investigated the effects of Cur and AP separately in the treatment groups. In the current study, Cur decreased IL-25 and IL-10 levels at a high dose of 32 µM/mL and a low dose did not show any difference in comparison to the BCM group. The protective effects of AP at 2 and 2.5 mg/mL were more significant than the cur groups.
MMP-2 shows an increase especially in inflammation as a regulatory modulator. Barbara Fingleton et al. investigated MMP-2’s role in chronic and acute inflammation. The MMP-2 level shows an increase in response to inflammation to start regeneration [
54]. This mechanism is a protective mechanism. The MMP-2 result in the BCM group shows an increase but treatment with AP more effectively decreased MMP-2. We think propolis’ different phenolic components have a role in low MMP-2 levels.
4. Materials and Methods
4.1. Chemicals and Reagents
All the reagents were of analytical grade and used without further purification. AP (Bee and You company, Nazobec (Farmamag İlaç San ve Tic A.Ş., Turkey)), Dulbecco-modified eagle medium (DMEM), fetal bovine serum (FBS), Antibiotic, and dimethyl sulfoxide (DMSO) were purchased from Sigma Aldrich (St. Louis, MO, USA). Primers and regents for gene expression and cDNA analyses by real-time PCR were obtained from Roche (Darmstadt, Germany).
4.2. GC/MS Spectrometry
Shimadzu/QP2010-ultra (Tokyo, Japan) was used for the analysis of the AP sample. Experimental conditions of the GCMS system were as follows. A REX 5MS column (30 m × 0.25 mm, 0.25 μm film thickness) was used. The column flow rate of the mobile phase (He) was set at 1 mL/min. In the GC part, the temperature was kept for 3 min at 40 °C and then increased to 250 °C at 10 °C/min intervals followed by 5 min at 150 °C. Finally, the temperature was increased to 300 °C at 18 °C/min for 42 min. The mass range (m/z) was determined as 30–500. The experiment was conducted in the Central Research Laboratory Application and Research Center (BARUM, Bilecik, Turkey)
4.3. Nasal Fibroblast Cell Culture
The fibroblast cell line (PCS-201-012, ATCC) was obtained from Bilecik University, Department of Medical Pharmacology. Cells were centrifuged at 1200 rpm for 5 min. They were suspended in fresh Dulbecco-modified eagle medium-F12 (DMEM-F12), fetal bovine serum (FBS) 10%, and antibiotics 1% (penicillin, streptomycin, and amphotericin B). Then, 48-well plates (5% CO2; 37°) were used for seeding.
4.4. MTT Assay
Briefly, cells were resuspended in fresh DMEM medium, 10% FBS, and 1% antibiotic (penicillin, streptomycin, and amphotericin B). Cells were then seeded in 96-well plates (Corning, NY, USA) as previously described and stored in an incubator (5% CO
2; 37 °C). After reaching 85% confluence on a 0.5 McFarland scale, the experiment was conducted with 14 groups. For the BCM toxicity model, BCM 11.90 µM/mL was exposed for 30 min; then, the treatment was added to the plate and the experiment was conducted for 24 h. The sample size was 8 (
n = 8). The control group received only medium and the BCM control group received BCM 11.90 µM/mL [
32]. BCM 11.90 µM/mL + AP 0.5 mg/mL, BCM 11.90 µM/mL + AP 1 mg/mL, BCM 11.90 µM/mL + AP 1.5 mg/mL, BCM 11.90 µM/mL + AP 2 mg/mL, BCM 11.90 µM/mL + AP 2.5 mg/mL, BCM 11.90 µM/mL + Cur 2 µM/mL, BCM 11.90 µM/mL + Cur 4 µM/mL, BCM 11.90 µM/mL + Cur 8 µM/mL, BCM 11.90 µM/mL + Cur 16 µM/mL, and BCM 11.90 µM/mL + Cur 32 µM/mL was administered for 24 h. Combination groups BCM 11.90 µM/mL + AP 1.5 mg/mL, BCM 11.90 µM/mL + AP 2 mg/mL, and BCM 11.90 µM/mL + AP 2.5 mg/mL for evaluation of a potential synergy effect were added for 24 h. At the end of the experiment, 10 μL of MTT solution (Sigma Aldrich, St. Louis, MO, USA) was added to each well plate and the samples were incubated for 4 h; 100 μL of DMSO (Millipore Sigma) was added to all wells to dissolve the Formazan crystals. The optical density of the solutions was read at 570 nm using a Multiskan™ GO microplate spectrophotometer (Thermo Fisher, Porto Salvo, Portugal) [
55].
4.5. TAC (Total Antioxidant Capacity) and TOS (Total Oxidant Status)
TAC and TOS evaluation was performed using a supernatant medium of each group at the end of the study. TAC level was measured by using the Rel Assay Total Antioxidant Capacity (Rel Assay Diagnostics, Gaziantep, Turkey) commercial kit. Briefly, the supernatant was used and the reagent was added according to the manufacturing protocol. The color change was evaluated by measuring at a wavelength of 660 nm for TAC and 530 nm for TOS. TAC results were expressed per µmol Trolox Equiv/mg protein. TOS results were expressed per µmol H
2O
2 Equiv/mg protein [
56].
4.6. LDH (Lactate dehydrogenase) and Gr (Glutathione reductase) Assay
LDH and Gr evaluation was performed using lysed cells of each group at the end of the study. The cells were lysed by 0.5% triton-x and then centrifuged at 5.000× g for 5 min to obtain the supernatant. The supernatants were taken and used for analysis. Lactate dehydrogenase (LDH) and Gr (Glutathione reductase) levels were assessed using the ELISA kit (Elabscience, United States Cat no., Houston, TX, USA) according to the manual of the manufacturer. At 450 nm, the optical densities of each sample were measured.
4.7. Real-Time PCR Analysis
4.7.1. RNA Isolation
mRNA isolation was obtained according to the procedure of Invitrogen™ Total RNA Isolation Kit (Catalog number: 4478545).
4.7.2. Gene Expression
Gene expression levels were examined in the effective groups. MMP-2, IL-25, VEGF, and IL-10 genes, 0.25 μL of right and left primer, 0.15 μL of the probe, 3 μL of cDNA, 3 master mix, and 12.75 μL of distilled water were added to each strip (tube) at this stage. The final volume was adjusted to 20 μL. After 600 s at 95 °C, 10 s at 95 degrees, and 30 s at 60 degrees, 45 cycles were made [
57].
β-actin
Forward: 5′-CCAACCGCGAGAAGATGA-3′
Reverse: 5′-CCAGAGGCGTACAGGGATAG-3′
MMP-2
Forward: 5′- CCGAGGACTATGACCGGGATAA-3
Reverse: 5′- CTTGTTGCCCAGGAAAGTGAAG-3′
IL-25
Forward: 5′-TGGCAATGATCGTGGGAACC-3′
Reverse: 5′-GAGAGATGGCCCTGCTGTTGA-3′
VEGF
Forward: 5′-ACCATGAACTTTCTGGTCTCTTG-3′
Reverse: 5′-TCGGGGTACTCCTGGAAGATG-3′
IL-10
Forward:5′-GGCGCTGTCATCGATTTCTT-3′
Reverse: 5′-TCTCTTGGAGCTTATTAAAGGCATTC-3′
FGF-2
Forward:5′-GTTGACCCTACCATGTTCCCTTG-3′
Reverse: 5′-GCCAGCAGCATCTATGGGAC-3′
HBD
Forward:5′-GACAGGTACGGCTGTCATCA-3′
Reverse: 5′-CAGCCTAAGGGTGGGAAAAT-3′
4.8. ELISA Test: Klotho, Galanin, mTOR, and STAT-3
The fibroblast cells were washed in iced PBS (pH 7.4) to thoroughly remove the supernatant residue. The cells were lysed and centrifuged at 12,000 RPM at 4 °C for 15 min. IL-25, IL-10, MMP-2, FGF-2, and VEGF BTLAB ELISAs (Cat No: E0054Hu, E0102Hu, E0315Ra, E1592Mo, and E0114Mo) were performed according to the kit protocol and each sample’s optical density was measured at 450 nm [
58].
4.9. Statistical Analysis
Data were statistically defined as mean and standard deviation (mean ± SD) for % area. One-way ANOVA and Tukey tests (SPSS 22.0) were performed to compare positive immunoreactive cells and immunopositive stained areas with healthy controls. As a result of the test, a value of p < 0.05 was considered significant and the data were presented as mean ± SD.
5. Conclusions
In our study, we evaluated MTT, TAC, TOS, LDH, and Gr parameters to evaluate whether BCM reduces cellular viability and increases oxidative stress in nasal fibroblasts. We used Cur and AP as treatments to reverse these effects. As seen so far, we have shown that cell viability is increased, and oxidative damage is prevented with the treatments we provide. We used the genetic analysis parameters IL-25, IL-10, MMP-2, FGF-2, HBD, and VEGF. Considering the results obtained, while BCM increased IL-25, IL-10, and MMP-2 levels, a decrease was detected in the expression levels of FGF-2 and VEGF. Contrary to these data, although AP and Cur show effective healing, AP has effectively decreased inflammation. Future Directions: This study is limited to in vitro analyses. In future studies, combined treatments against BCM toxicity (AP 2 µg/mL + Cur 16 µM/mL) can be validated with in vivo experiments and their clinical applicability can be tested.