1. Introduction
A wound is defined as a disruption in the continuity of cellular, anatomical, and functional structures of living tissue, caused by various factors, such as physical, chemical, thermal, microbial, or immunological injury. This disruption typically begins with a breach in epithelial cohesion, which may extend to compromise the structural and functional stability of the underlying tissue. Chronic wounds are characterized by a prolonged healing process, often due to impaired angiogenesis, innervation, or cellular migration. These wounds, particularly those associated with cutaneous ulceration and systemic conditions such as autoimmune or inflammatory disorders, pose significant treatment challenges. Additionally, they are often colonized by antibiotic-resistant bacteria, requiring effective management of the underlying condition before considering definitive surgical intervention [
1,
2,
3]. Skin and soft tissue infections frequently involve cellulitis, which is characterized by localized erythema, edema, and increased local temperature. This condition occurs due to bacterial entry through disruptions in the skin barrier, resulting in an inflammatory response [
4,
5,
6]. Cellulitis predominantly affects middle-aged and elderly individuals, with various factors heightening the risk. Trauma-related disruption of the skin barrier, inflammatory skin conditions like eczema and psoriasis, edema from impaired lymphatic drainage or venous insufficiency, and obesity all contribute to susceptibility [
7,
8,
9,
10,
11]. Studies showed that 92% of lymphedema-related hospital admissions are associated with cellulitis [
12]. Immunosuppression, unnoticed skin breaks, pre-existing infections, and close contact with individuals carrying methicillin-resistant
Staphylococcus aureus (MRSA) represent additional risks for purulent infections [
13]. Complications arising from cellulitis and abscesses can lead to serious systemic conditions, such as bacteremia, endocarditis, septic arthritis, osteomyelitis, metastatic infections, sepsis, and toxic shock syndrome. These severe outcomes underscore the importance of recognizing and addressing predisposing factors, as outlined, to prevent the escalation of localized infections into more life-threatening conditions [
4]. The primary cause of cellulitis is beta-hemolytic streptococci, particularly group A
Streptococcus, with
Staphylococcus aureus, including methicillin-resistant strains, being a less common but notable pathogen. In some cases, Gram-negative bacilli may also be implicated [
14,
15,
16,
17].
Chronic venous insufficiency, which leads to recurrent edema, is a key factor predisposing individuals to recurrent cellulitis, especially when accompanied by venous leg ulcers (VLUs). VLUs, the most common chronic wound, affecting approximately 4% of the population, typically occur in the lower extremities and are associated with high recurrence rates, prolonged disability, and psychosocial burden [
18]. These ulcers, which vary in size and severity, can produce excessive exudate, leading to skin irritation and further complications like cellulitis [
19,
20,
21,
22,
23,
24]. Venous stasis ulcers constitute over half of all chronic lower-limb wounds. These wounds are often colonized by pathogens such as
Staphylococcus aureus,
Pseudomonas aeruginosa, and β-hemolytic streptococci, which hinder healing by inducing inflammation. Bacterial presence attracts leukocytes, leading to an increase in inflammatory cytokines, proteases, and reactive oxygen species (ROS), thus perpetuating inflammatory cycles that impede wound repair [
25]. Signs of infection, such as localized heat, erythema, or fever, require prompt diagnosis and treatment in order to prevent cellulitis from progressing to more severe complications, such as bacteremia or sepsis [
26].
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that primarily affects regions characterized by a high density of hair follicles, with prevailing hypotheses suggesting that it arises from follicular occlusion [
27]. European studies, which include undiagnosed cases, typically estimate a prevalence of 1% or higher, indicating that the condition is relatively common [
28]. The disease pathogenesis is likely multifactorial, with genetic predisposition, cellular and immune factors, nutrition, obesity, and smoking—as a risk factor for many chronic conditions, including psoriasis—all playing potential roles [
29,
30]. The clinical manifestations of HS vary widely, ranging from inflamed nodules and abscesses to draining sinus tracts, often resulting in significant pain, malodor, and scarring, which adversely affect the quality of life [
31,
32]. The involvement of bacteria in HS remains a subject of contention; early lesions are generally sterile, whereas older or ruptured lesions may be colonized by various microorganisms, including staphylococci, streptococci, and other facultative anaerobes. Although these bacteria may signify secondary infections or mere contaminants, some theories postulate that bacterial biofilms may exacerbate the chronic inflammatory response associated with HS [
33]. In cases that are severe or inadequately managed, bacterial infections, including cellulitis, represent notable complications [
34,
35,
36,
37,
38,
39].
Bacterial resistance to antimicrobials can be either intrinsic or acquired. Intrinsic resistance is a natural trait exhibited by nearly all members of a species, as seen in
Klebsiella pneumoniae’s resistance to ampicillin, eliminating the need for susceptibility testing [
40]. Acquired resistance, however, develops in response to antimicrobial exposure in previously susceptible bacteria, often through chromosomal mutations or horizontal gene transfer via plasmids, integrons, transposons, or transformation. Unlike intrinsic resistance, acquired resistance varies among isolates, making susceptibility testing essential for therapeutic decisions [
41]. Resistance expression may be constitutive, occurring continuously, or inducible, triggered by specific agents; for example, third-generation cephalosporins can induce AmpC beta-lactamase production in some
Enterobacterales [
42]. Additionally, heteroresistance, a variable expression of resistance within bacterial subpopulations, poses challenges for detection, sometimes requiring susceptibility tests with higher inocula to identify resistant subpopulations accurately, as seen in vancomycin-intermediate
Staphylococcus aureus [
43]. The terms “susceptible”, “intermediate”, and “resistant” categorize microorganisms based on their responses to antimicrobial agents. Susceptible organisms are effectively inhibited or killed by antibiotics at standard dosing levels, while resistant organisms fail to respond even at concentrations exceeding safe or achievable levels in the body. The intermediate category reflects an uncertain response, where higher doses or optimal conditions might render the antibiotic effective [
44]. These classifications are crucial for optimizing treatment and understanding overall resistance patterns within populations. Moreover, standardizing antimicrobial susceptibility testing methods and adopting harmonized evaluation criteria are essential for accurate classification. Such standardization enhances clinical decision making, supports resistance monitoring, and ensures consistency in reporting across laboratories and regions [
44,
45].
3. Discussion
Our study provides valuable insights into the microbial populations associated with chronic wounds, particularly in patients with hidradenitis suppurativa (HS) and venous ulcers (VU). We observed patterns that align with findings from the literature but also revealed unique differences in microbial diversity and antibiotic resistance profiles.
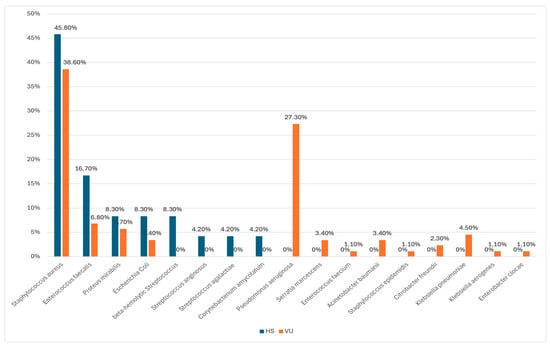
Our results show that
Staphylococcus aureus was the most frequently identified pathogen in both groups (45.8% in HS; 38.6% in VU). This aligns with Wysocki et al., who identified
S. aureus as a dominant pathogen in venous ulcers, and Wolcott et al., who similarly found
S. aureus to be a prominent chronic wound pathogen [
46,
47]. Furthermore, Wong et al. reported comparable prevalence rates for
S. aureus (23.3%) in chronic wounds, further supporting our findings [
48]. In line with our study, Katoulis et al. also found a high prevalence of
S. aureus in HS wounds [
49]. However, our observed prevalence of
S. aureus (45.8%) in HS patients contrasts with the very high rates reported by Miller et al. for venous leg ulcers (up to 90%), suggesting that the prevalence of this pathogen might vary significantly depending on wound type and demographic factors [
50]. Moreover,
Staphylococcus aureus, particularly methicillin-resistant
S. aureus (MRSA), is a major pathogen responsible for a wide range of infections, from mild skin conditions to severe diseases like pneumonia and endocarditis. The bacterium has developed resistance through various mechanisms, including β-lactamase production, efflux systems, and biofilm formation, which complicate treatment. Resistance to penicillin exceeds 90%, and the emergence of vancomycin-resistant
S. aureus (VRSA) further limits therapeutic options. The global prevalence of MRSA is rising, particularly in developing countries where poor hygiene and overcrowded conditions contribute to the spread of community-acquired MRSA (CA-MRSA). In contrast, hospital-acquired MRSA (HA-MRSA) infections are declining in developed regions due to improved healthcare practices. However, the increasing prevalence of multidrug-resistant strains and the emergence of severe infections underscore the urgent need for continued surveillance and enhanced antimicrobial stewardship worldwide [
51,
52,
53]. Similar to our results, MRSA, as reported in China, showed higher resistance to various antibiotics, including macrolides, quinolones, and aminoglycosides, while remaining highly sensitive to vancomycin and teicoplanin. Our study also found significant resistance to clindamycin and erythromycin, with resistance rates of 53.5% and 55%, respectively. These findings align with global trends, where MRSA strains generally exhibit moderate resistance to several antibiotics but remain responsive to vancomycin and teicoplanin [
52].
In addition to their antimicrobial effects, antibiotics have significant anti-inflammatory properties, which are particularly relevant in managing hidradenitis suppurativa (HS). These properties extend beyond pathogen elimination, targeting the inflammatory pathways that exacerbate HS. Antibiotics are considered first-line treatment due to their antimicrobial, anti-inflammatory, and immunomodulatory effects, underscoring the importance of careful selection to balance efficacy with their role in modulating inflammation. However, the growing challenge of antimicrobial resistance, particularly related to biofilm production, highlights the need to refine treatment approaches and reassess the clinical value of swab-guided antibiotic therapy, warranting further evaluation in randomized clinical trials [
54].
Pseudomonas aeruginosa is a major cause of severe hospital-acquired infections and poses a significant global health threat due to its high levels of antimicrobial resistance (AMR). Resistance mechanisms, including efflux pump overexpression, porin mutations, and β-lactamase production, hinder the effectiveness of multiple antibiotics, complicating treatment, especially in immunocompromised patients. The pathogen’s ability to acquire resistance genes through horizontal gene transfer further exacerbates the challenge. AMR in
P. aeruginosa is a critical concern worldwide. Addressing this growing issue requires enhanced surveillance, improved healthcare infrastructure, and targeted treatment strategies to combat the spread of multidrug-resistant strains and protect public health [
51,
53,
55,
56].
A striking difference in our study was the absence of
Pseudomonas aeruginosa in HS patients, while it was identified in 27.3% of VU patients. This aligns with findings from Bialasik, who also reported a higher prevalence of
P. aeruginosa in venous leg ulcers [
57]. Moreover, our study also aligns with findings by Rahim and Dowd et al., where
P. aeruginosa was less prevalent in HS than in other chronic wound types, further supporting the hypothesis that microbial populations in HS may differ significantly from those in other wound types [
58,
59]. Notably,
Pseudomonas aeruginosa was present in 31.7% of cases analyzed by Benzecry et al., suggesting that local factors might influence microbial populations in HS wounds [
60]. Furthermore, our results resonate with those of Nikolakis et al., who found a greater prevalence of
P. aeruginosa in venous ulcers than in HS wounds, potentially influenced by differences in wound environments and host factors [
29]. In contrast, studies by Brook et al. and Jockenhöfer et al. identified
P. aeruginosa across both VU and HS cases [
61,
62]. Our study highlights the need to consider such variations when diagnosing and treating chronic wounds.
The antimicrobial resistance (AMR) profiles of
Pseudomonas aeruginosa in this study align with global concerns about the increasing resistance of this pathogen to multiple antibiotics. Resistance in our cohort was highest to oxacillin and ceftolozane-tazobactam, which is consistent with findings from studies in other regions, such as those in China, where resistance to carbapenems like imipenem and meropenem has fluctuated over the years. In China, resistance to imipenem dropped from 30.8% in 2010 to 22.1% in 2022, while meropenem resistance decreased from 25.8% to 17.6% in the same period, although carbapenem-resistant
P. aeruginosa (CRPA) remained a concern, particularly in Zhejiang Province, where resistance reached 38.67% by 2017 [
52]. Similarly, in the Arabian Gulf countries,
P. aeruginosa demonstrated significant resistance to meropenem (10.3–45.7%) but remained largely susceptible to colistin [
63]. Our study also observed high susceptibility to gentamicin, amikacin, and tobramycin, which contrasts with the findings of Telling et al., where resistance to gentamicin was present in 19.6% of isolates [
64]. Additionally, the prevalence of cefiderocol non-susceptibility (CFDC-NS) in
P. aeruginosa has been generally low in global studies but notably higher in carbapenem-resistant strains, highlighting the challenge of treating resistant infections with newer agents [
65]. Collectively, these studies underscore the global variability in resistance patterns, emphasizing the urgent need for continuous regional surveillance and standardized testing to guide treatment strategies and combat the growing threat of multidrug-resistant
P. aeruginosa.Interestingly, our study identified beta-hemolytic
Streptococcus and
Corynebacterium amycolatum in the HS subpopulation only. This finding is consistent with Benzecry et al., who identified similar bacterial species in HS patients, though with differences in prevalence rates. Specifically, while we found beta-hemolytic
Streptococcus in 8.3% of HS cases, Benzecry et al. reported
Streptococcus spp. in approximately 5.3% of their cohort [
60]. In contrast, Eriksson et al., found that
Streptococcus species were more likely to colonize venous ulcers due to the unique inflammatory environment in these lesions [
66].
In terms of antibiotic resistance, our findings show moderate resistance to multiple antibiotics among the most frequently identified pathogens.
S. aureus exhibited resistance to an average of 1.64 ± 1.71 antibiotics, and
Proteus mirabilis demonstrated resistance to 2.29 ± 1.38 antibiotics. This is in line with studies by Ayobami et al. and Bettoli et al., who noted higher resistance rates in MRSA and
P. aeruginosa isolates in North America and Asia, respectively [
67,
68]. However, the resistance patterns in our study did not reach statistical significance, potentially due to the relatively small sample size or the inclusion of both sensitive and resistant strains in the analysis.
Notably, we found that 100% of the isolates tested were sensitive to tigecycline, teicoplanin, and vancomycin. These findings are consistent with reports from Wysocki et al., who also noted high sensitivity of chronic wound pathogens to these antibiotics, particularly in cases involving MRSA and multidrug-resistant strains [
69]. Our study also observed moderate resistance to common antibiotics like erythromycin and clindamycin, which is consistent with findings by Hessam et al. regarding
S. aureus resistance in HS infections [
70].
In the HS group, we also observed a significant prevalence of
Enterococcus faecalis, with 9 out of 10 cases showing sensitivity to all tested antibiotics. This shows that while
Enterococcus species are commonly found in chronic wounds, they did not exhibit high resistance in the cohort we studied. This finding contrasts with studies in other regions, where
Enterococcus species showed higher resistance profiles, especially in the context of polymicrobial infections [
68,
70].
Furthermore, our findings mirror the microbial diversity reported by Dowd et al., who highlighted
Staphylococcus aureus and
Pseudomonas aeruginosa as dominant pathogens in chronic wounds but also showed that Enterobacteriaceae, including
E. coli and
Proteus mirabilis, are common in venous ulcers [
59]. The research conducted by Thomas et al. on wound microbiomes in oxygen-deficient environments further supports our observation of anaerobic conditions being more pronounced in HS lesions, which may contribute to the unique microbial profiles observed in these wounds [
71].
The antimicrobial resistance profiles observed in our study align with global trends, particularly regarding
Escherichia coli,
Enterococcus faecalis,
Proteus mirabilis, and
Streptococcus species. For
E. coli, our study found high susceptibility to gentamicin and co-trimoxazole, with moderate resistance to ampicillin, similar to the resistance patterns reported by Abayneh et al., who found high resistance rates to ampicillin (89%) and co-trimoxazole (83%) [
72]. In a study conducted in China,
E. coli also showed moderate resistance to ciprofloxacin (61.4%) and cefepime (25.1%) but remained highly susceptible to carbapenems, a trend reflected in our study where
E. coli isolates were fully susceptible to meropenem and ertapenem, aligning with findings published by Luo et al. [
52].
Enterococcus faecalis in our cohort exhibited a similar trend, with high susceptibility to ampicillin and vancomycin, contrasting with the growing global concerns of vancomycin-resistant enterococci (VRE), highlighted by Zhu et al. [
73]. Our findings for
Proteus mirabilis were consistent with the study conducted by Torrens et al., which showed complete susceptibility to amikacin, ceftazidime, ceftriaxone, meropenem, and ertapenem while demonstrating mixed susceptibility to co-trimoxazole, a trend that was also observed in our cohort [
74]. Furthermore, beta-hemolytic
Streptococcus isolates in our study showed 100% susceptibility to penicillin, erythromycin, co-trimoxazole, and rifampicin while demonstrating mixed resistance to tetracycline and clindamycin, a pattern similar to that reported by Torrens et al. [
74]. Therefore, regional and global antimicrobial surveillance are of the utmost importance in order to better understand and combat the rise of multidrug-resistant pathogens.
In summary, our study showed Staphylococcus aureus and Pseudomonas aeruginosa as being major pathogens in chronic wounds, with distinct microbial compositions observed in HS compared to VU. We also found moderate antibiotic resistance, particularly among S. aureus and P. mirabilis, underscoring the need for personalized treatment strategies. The diversity of bacterial species suggests that wound microbiomes are highly patient-specific and also region-specific. These findings highlight the critical need for continuous surveillance of microbial populations and antibiotic resistance patterns in order to optimize the management of chronic wounds.
However, this study has several limitations that should be considered. First, the relatively small sample size and lack of diversity in the patient population may limit the generalizability of the findings to broader populations. Additionally, the cross-sectional design restricts the possibility to further observe changes in microbial populations over time. Using culture-based methods may have caused certain unculturable bacteria to be missed, and antibiotic resistance testing was limited to a standard panel, thus potentially underestimating the full spectrum of resistance profiles. Furthermore, the study did not include a control group of healthy individuals, which would have helped distinguish between pathogenic and non-pathogenic bacteria. Lastly, variations in antibiotic use and wound-care practices across patients could have influenced microbial diversity and resistance patterns.