1. Introduction
2. Results
There was a significant difference in the presence of polymicrobial infection between the three groups (p = 0.013). Polymicrobial infection was more likely in osteomyelitis (36.2%) compared to prosthetic joint infection (21.6%) (p = 0.012), but there was no difference in the polymicrobial infection rates between fracture-related infection and the other two groups (p = 0.171 between FRI and PJI and p = 1.0 between FRI and OM). Of the patients with fracture-related infection, 37/115 (32.2%) had polymicrobial infection. Monomicrobial infection was relatively more common in FRIs presenting early, within 4 weeks of fracture (14/21; 66.6%), or in chronic cases with a duration of infection of more than 12 weeks (59/83; 71.1%). Polymicrobial infection was most common in FRIs with a shorter duration (4–12 weeks) (6/11; 54.5%).
Prosthetic Material Compared to No Prosthetic Material
Staphylococcus aureus was more likely in osteomyelitis than in infections with prosthetic material (62.6% versus 49.1%, p = 0.006). The presence of polymicrobial infection was 36.2% in osteomyelitis compared to 26% in patients with prosthetic material (p = 0.024). When adjusted for confounders, there was no difference in the presence of Staphylococcus aureus (OR = 0.654; 95% CI 0.402–1.066; p = 0.088) or polymicrobial infection (OR = 0.687; 95% CI 0.410–1.150; p = 0.153).
3. Discussion
Empiric regimes have often been based on the assumption that bone and joint infections are predominantly Gram-positive, with limited need for Gram-negative cover. Also, regimes have been different in Trauma Units, which treat fractures and fracture-related infections, compared to elective Orthopaedic Units, where prosthetic joint replacement is performed, and osteomyelitis is treated. Multiple antimicrobial policies can be confusing for treating staff.
This study suggests that the microbiology is not significantly different, and that polymicrobial infection, including Gram-negative organisms, is a common occurrence. Simplifying empiric antimicrobial regimes based on these findings may improve antimicrobial stewardship and compliance in hospitals.
3.1. Polymicrobial Infections
3.2. Local Antibiotic Treatment Implications
3.3. Limitations
The main limitation of this study was that it is a single-centre retrospective review. Thus, the microbiological epidemiology is representative only of the single centre and may not be able to be extrapolated to other regions. However, as a tertiary referral centre, the Bone Infection Unit receives patients referred from all over the United Kingdom and likely represents a wider epidemiological base than just the local catchment. Additionally, prior antibiotic treatment history was not consistently available to be included in this study and may have affected the culture results.
4. Materials and Methods
This was a retrospective observational cohort study at Oxford University Hospital Bone Infection Unit. The Bone Infection Unit is a specialized unit treating adult patients from the United Kingdom with musculoskeletal infections, including fracture-related infection, prosthetic joint infections and haematogenous infections of the bones, joints and spine.
4.1. Recruitment and Inclusion Criteria
The medical and surgical records and laboratory details of all patients who were admitted for treatment to the Bone Infection Unit between January 2019 and September 2022 were reviewed. We identified all consecutive patients with a diagnosis of prosthetic joint infection, osteomyelitis and fracture-related infection who were also treated surgically. Individual patients were only included once. Recurrent infections were not included. Patients who were under 18 years old at the time of treatment were excluded. Patients were only included if they had implantation of antibiotic carriers as part of their surgical management.
4.2. Microbial Sampling
4.3. Data Collection
4.4. Data Management and Analysis
Analysis was performed using IBM SPSS v29. The difference in the microbiological organism between the three groups (osteomyelitis, fracture-related infection and prosthetic joint infection) were compared using a one-way ANOVA. Post hoc testing used an independent samples t test, and Bonferroni correction was used to determine the difference. Potential confounders considered for inclusion in the regression models were age, BMI, the location of injury, JS-BACH score and symptom duration. Each potential confounder was examined separately by univariate analysis to determine their association with the outcome variable. Confounders whose association with the outcome variable had a p value < 0.2 were included in the multivariate model.
5. Conclusions
Causative pathogens are similar across bone and joint infections and are independent of the presence of prosthetic material. There was no significant difference in the identification, presence of polymicrobial infection or gentamicin and vancomycin resistance in organisms that were isolated in osteomyelitis or fracture-related infection compared to prosthetic joint infection. This may have implications for the development and standardization of empiric antibiotic regimens and local antibiotic therapy in the management of bone and joint infections.
Author Contributions
Conceptualization, M.M.; methodology, A.U., M.M., M.S. and B.Y.; formal analysis, A.U. and M.M.; investigation, A.U and M.M.; data curation, A.U. and M.M.; writing—original draft preparation, A.U. and M.M.; writing—review and editing, A.U., M.M., M.S. and B.Y.; supervision, M.M., B.Y. and M.S.; All authors have read and agreed to the published version of the manuscript.
Funding
AU received a Royal Australian College of Physicians Travel Grant to undertake a clinical fellowship at the Oxford Bone Infection Unit, Nuffield Orthopaedic Centre, Oxford, UK. No other funding was received for this study.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by our Institutional Governance Board as a Quality Improvement Project (OUH 2023/8356). No ethical committee review was required.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy reasons.
Acknowledgments
We would like to thank the staff of the Oxford Bone Infection Unit and The John Radcliffe Hospital Microbiology Laboratory for their dedication to the care of these patients.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carter, K.J.; Yeager, M.T.; Rutz, R.W.; Benson, E.M.; Gross, E.G.; Campbell, C.; Johnson, J.P.; Spitler, C.A. Lower Extremity Amputation in Fracture Related Infection. J. Orthop. Trauma 2022, 38, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, N.R.; Mechlenburg, I.; Søballe, K.; Lange, J. Patient-reported quality of life and hip function after 2-stage revision of chronic periprosthetic hip joint infection: A cross-sectional study. Hip Int. 2018, 28, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, K.; Clauss, M.; Joeris, A.; Kates, S.; Morgenstern, M. Health-related quality of life and mental health in patients with major bone and joint infections. Bone Jt. Open 2024, 5, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.; Kuehl, R.; Moriarty, T.; Richards, R.; Verhofstad, M.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522. [Google Scholar] [CrossRef]
- Ahmed, S.; Haddad, F. Prosthetic joint infection. Bone Jt. Res. 2019, 8, 570–572. [Google Scholar] [CrossRef]
- Dudareva, M.; Kümin, M.; Vach, W.; Kaier, K.; Ferguson, J.; McNally, M.; Scarborough, M. Short or Long Antibiotic Regimes in Orthopaedics (SOLARIO): A randomised controlled open-label non-inferiority trial of duration of systemic antibiotics in adults with orthopaedic infection treated operatively with local antibiotic therapy. Trials 2019, 20, 693. [Google Scholar] [CrossRef]
- Rupp, M.; Walter, N.; Popp, D.; Hitzenbichler, F.; Heyd, R.; Geis, S.; Kandulski, M.; Thurn, S.; Betz, T.; Brochhausen, C. Multidisciplinary treatment of fracture-related infection has a positive impact on clinical outcome—A retrospective case control study at a tertiary referral center. Antibiotics 2023, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Bezstarosti, H.; Van Lieshout, E.; Voskamp, L.; Kortram, K.; Obremskey, W.; McNally, M.; Metsemakers, W.-J.; Verhofstad, M. Insights into treatment and outcome of fracture-related infection: A systematic literature review. Arch. Orthop. Trauma Surg. 2019, 139, 61–72. [Google Scholar] [CrossRef]
- Australian Commission on Safety and Quality in Healthcare. What Are Antibiograms? Australian Commission on Safety and Quality in Healthcare: Sydney, Australia, 2021. [Google Scholar]
- Corrigan, R.A.; Sliepen, J.; Dudareva, M.; IJpma, F.F.; Govaert, G.; Atkins, B.L.; Rentenaar, R.; Wouthuyzen-Bakker, M.; McNally, M. Causative pathogens do not differ between early, delayed or late fracture-related infections. Antibiotics 2022, 11, 943. [Google Scholar] [CrossRef]
- Sigmund, I.K.; McNally, M.A. Diagnosis of bone and joint infections. Orthop. Trauma 2019, 33, 144–152. [Google Scholar] [CrossRef]
- Peel, T.N.; Dylla, B.L.; Hughes, J.G.; Lynch, D.T.; Greenwood-Quaintance, K.E.; Cheng, A.C.; Mandrekar, J.N.; Patel, R. Improved diagnosis of prosthetic joint infection by culturing periprosthetic tissue specimens in blood culture bottles. MBio 2016, 7, e01776. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, E.J.; Stephens-Shields, A.J.; Newcomb, C.W.; Silibovsky, R.; Nelson, C.L.; O’Donnell, J.A.; Glaser, L.J.; Hsieh, E.; Hanberg, J.S.; Tate, J.P. Incidence, microbiological studies, and factors associated with prosthetic joint infection after total knee arthroplasty. JAMA Netw. Open 2023, 6, e2340457. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Ricciardi, B.F.; Bentley, K.L.d.M.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef]
- Depypere, M.; Kuehl, R.; Metsemakers, W.-J.; Senneville, E.; McNally, M.A.; Obremskey, W.T.; Zimmerli, W.; Atkins, B.L.; Trampuz, A. Recommendations for systemic antimicrobial therapy in fracture-related infection: A consensus from an international expert group. J. Orthop. Trauma 2020, 34, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.B.G.; Patel, R.; Abdel, M.P.; Berbari, E.F.; Tande, A.J. Microbiology of hip and knee periprosthetic joint infections: A database study. Clin. Microbiol. Infect. 2022, 28, 255–259. [Google Scholar] [CrossRef]
- Depypere, M.; Sliepen, J.; Onsea, J.; Debaveye, Y.; Govaert, G.A.; IJpma, F.F.; Zimmerli, W.; Metsemakers, W.-J. The microbiological etiology of fracture-related infection. Front. Cell. Infect. Microbiol. 2022, 12, 934485. [Google Scholar] [CrossRef]
- Li, H.-K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M. Oral versus intravenous antibiotics for bone and joint infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef]
- Conterno, L.O.; Turchi, M.D. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst. Rev. 2013, 9, CD004439. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Arvieux, C.; Brunschweiler, B.; Touchais, S.; Ansart, S.; Bru, J.-P.; Oziol, E.; Boeri, C.; Gras, G.; Druon, J. Antibiotic therapy for 6 or 12 weeks for prosthetic joint infection. N. Engl. J. Med. 2021, 384, 1991–2001. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Reinecke, P.; Morovic, P.; Niemann, M.; Renz, N.; Perka, C.; Trampuz, A.; Meller, S. Adverse Events Associated with Prolonged Antibiotic Therapy for Periprosthetic Joint Infections—A Prospective Study with a Special Focus on Rifampin. Antibiotics 2023, 12, 1560. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Bernard, L.; Belaieff, W.; Gamulin, A.; Racloz, G.; Emonet, S.; Lew, D.; Hoffmeyer, P.; Uçkay, I. Epidemiology of adverse events and Clostridium difficile-associated diarrhea during long-term antibiotic therapy for osteoarticular infections. J. Infect. 2013, 67, 433–438. [Google Scholar] [CrossRef]
- Shah, N.B.; Hersh, B.L.; Kreger, A.; Sayeed, A.; Bullock, A.G.; Rothenberger, S.D.; Klatt, B.; Hamlin, B.; Urish, K.L. Benefits and adverse events associated with extended antibiotic use in total knee arthroplasty periprosthetic joint infection. Clin. Infect. Dis. 2020, 70, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Moran, E.; Masters, S.; Berendt, A.; McLardy-Smith, P.; Byren, I.; Atkins, B. Guiding empirical antibiotic therapy in orthopaedics: The microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J. Infect. 2007, 55, 1–7. [Google Scholar] [CrossRef]
- Patel, K.H.; Gill, L.I.; Tissingh, E.K.; Galanis, A.; Hadjihannas, I.; Iliadis, A.D.; Heidari, N.; Cherian, B.; Rosmarin, C.; Vris, A. Microbiological profile of fracture related infection at a UK Major Trauma Centre. Antibiotics 2023, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Baertl, S.; Walter, N.; Hitzenbichler, F.; Ehrenschwender, M.; Alt, V. Is there a difference in microbiological epidemiology and effective empiric antimicrobial therapy comparing fracture-related infection and periprosthetic joint infection? A retrospective comparative study. Antibiotics 2021, 10, 921. [Google Scholar] [CrossRef]
- Dudareva, M.; Barrett, L.; Morgenstern, M.; Atkins, B.; Brent, A.; McNally, M. Providing an evidence base for tissue sampling and culture interpretation in suspected fracture-related infection. JBJS 2021, 103, 977–983. [Google Scholar] [CrossRef]
- Dudareva, M.; Hotchen, A.J.; Ferguson, J.; Hodgson, S.; Scarborough, M.; Atkins, B.L.; McNally, M.A. The microbiology of chronic osteomyelitis: Changes over ten years. J. Infect. 2019, 79, 189–198. [Google Scholar] [CrossRef]
- Triffault-Fillit, C.; Ferry, T.; Laurent, F.; Pradat, P.; Dupieux, C.; Conrad, A.; Becker, A.; Lustig, S.; Fessy, M.-H.; Chidiac, C. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: A prospective cohort study. Clin. Microbiol. Infect. 2019, 25, 353–358. [Google Scholar] [CrossRef]
- Lemaignen, A.; Bernard, L.; Marmor, S.; Ferry, T.; Grammatico-Guillon, L.; Astagneau, P. Epidemiology of complex bone and joint infections in France using a national registry: The CRIOAc network. J. Infect. 2021, 82, 199–206. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. 2024. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 4 October 2024).
- Unsworth, A.; Young, B.; Ferguson, J.; Scarborough, M.; McNally, M. Local Antimicrobial Therapy with Combined Aminoglycoside and Vancomycin Compared to Aminoglycoside Monotherapy in the Surgical Management of Osteomyelitis and Fracture-Related Infection. Antibiotics 2024, 13, 703. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Bourget-Murray, J.; Hotchen, A.J.; Stubbs, D.; McNally, M. A comparison of clinical and radiological outcomes between two different biodegradable local antibiotic carriers used in the single-stage surgical management of long bone osteomyelitis. Bone Jt. Res. 2023, 12, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Govaert, G.A.; Kuehl, R.; Atkins, B.L.; Trampuz, A.; Morgenstern, M.; Obremskey, W.T.; Verhofstad, M.H.; McNally, M.A.; Metsemakers, W.-J. Diagnosing fracture-related infection: Current concepts and recommendations. J. Orthop. Trauma 2020, 34, 8–17. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.; Sousa, R.; Wouthuyzen-Bakker, M.; Chen, A.F.; Soriano, A.; Vogely, H.C.; Clauss, M.; Higuera, C.A.; Trebše, R. The EBJIS definition of periprosthetic joint infection: A practical guide for clinicians. Bone Jt. J. 2021, 103, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sandbakken, E.T.; Witsø, E.; Sporsheim, B.; Egeberg, K.W.; Foss, O.A.; Hoang, L.; Bjerkan, G.; Løseth, K.; Bergh, K. Highly variable effect of sonication to dislodge biofilm-embedded Staphylococcus epidermidis directly quantified by epifluorescence microscopy: An in vitro model study. J. Orthop. Surg. Res. 2020, 15, 522. [Google Scholar] [CrossRef]
- Young, B.C.; Dudareva, M.; Vicentine, M.P.; Hotchen, A.J.; Ferguson, J.; McNally, M. Microbial persistence, replacement and local antimicrobial therapy in recurrent bone and joint infection. Antibiotics 2023, 12, 708. [Google Scholar] [CrossRef]
- Morgenstern, M.; Athanasou, N.A.; Ferguson, J.Y.; Metsemakers, W.-J.; Atkins, B.L.; McNally, M.A. The value of quantitative histology in the diagnosis of fracture-related infection. Bone Jt. J. 2018, 100, 966–972. [Google Scholar] [CrossRef]
- Sigmund, I.K.; Yeghiazaryan, L.; Luger, M.; Windhager, R.; Sulzbacher, I.; McNally, M.A. Three to six tissue specimens for histopathological analysis are most accurate for diagnosing periprosthetic joint infection. Bone Jt. J. 2023, 105, 158–165. [Google Scholar] [CrossRef]
- Minassian, A.M.; Newnham, R.; Kalimeris, E.; Bejon, P.; Atkins, B.L.; Bowler, I.C. Use of an automated blood culture system (BD BACTEC™) for diagnosis of prosthetic joint infections: Easy and fast. BMC Infect. Dis. 2014, 14, 233. [Google Scholar] [CrossRef]
- Michel, J.-P.; Klopfenstein, C.; Hoffmeyer, P.; Stern, R.; Grab, B. Hip fracture surgery: Is the pre-operative American Society of Anesthesiologists (ASA) score a predictor of functional outcome? Aging Clin. Exp. Res. 2002, 14, 389–394. [Google Scholar] [CrossRef]
- Hotchen, A.J.; Wismayer, M.G.; Robertson-Waters, E.; McDonnell, S.M.; Kendrick, B.; Taylor, A.; Alvand, A.; McNally, M. The Joint-Specific BACH classification: A predictor of outcome in prosthetic joint infection. EClinicalMedicine 2021, 42, 101192. [Google Scholar] [CrossRef] [PubMed]
- Hotchen, A.J.; Dudareva, M.; Ferguson, J.Y.; Sendi, P.; McNally, M.A. The BACH classification of long bone osteomyelitis. Bone Jt. Res. 2019, 8, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.N.; Chambers, H.F.; Saag, M.S.; Pavia, A.T.; Boucher, H.W.; Black, D.; Freedman, D.O.; Kim, K.; Schwart, B.S. The Sanford Guide to Antimicrobial Therapy 2023; Antimicrobial Therapy, Inc.: Sperryville, VA, USA, 2023. [Google Scholar]
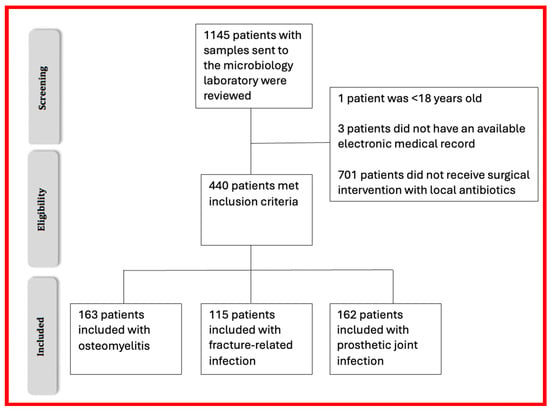
Patient inclusion flow diagram.
Figure 1.
Patient inclusion flow diagram.
Table 1.
Baseline patient demographics.
Table 1.
Baseline patient demographics.
| Characteristic | Osteomyelitis (n = 163) | Fracture-Related Infection (n = 115) | Prosthetic Joint Infection (n = 162) | |||
|---|---|---|---|---|---|---|
| Median | IQR * | Median | IQR * | Median | IQR * | |
| Age (years) | 56 | 37–66 | 56 | 41–64 | 70 | 60–78 |
| ASA * | 2 | 1–3 | 2 | 1–3 | 3 | 2–3 |
| BMI * | 26 | 22–31 | 27 | 24–31 | 30 | 25–36 |
| n | % | n | % | n | % | |
| Sex (Male) | 125 | 76.7 | 75 | 65.2 | 72 | 44.4 |
| Site of Infection | ||||||
| Long Bone | 135 | 82.8 | 91 | 79.1 | 0 | 0 |
| Hand and wrist | 5 | 3.1 | 1 | 0.9 | 1 | 0.6 |
| Foot and ankle | 16 | 9.8 | 10 | 8.7 | 2 | 1.2 |
| Hip | 2 | 1.2 | 0 | 0 | 79 | 48.8 |
| Knee | 1 | 0.6 | 1 | 0.9 | 75 | 46.3 |
| Spine | 3 | 1.8 | 12 | 10.4 | 0 | 0 |
| Elbow | 0 | 0 | 0 | 0 | 3 | 1.9 |
| Shoulder | 1 | 0.6 | 0 | 0 | 2 | 1.2 |
Table 2.
Infection characteristics.
Table 2.
Infection characteristics.
| Characteristic | Osteomyelitis (n = 163) | Fracture-Related Infection (n = 115) | Prosthetic Joint Infection (n = 162) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| JS-BACH * | ||||||
| Uncomplicated | 57 | 35.0 | 40 | 34.8 | 27 | 16.7 |
| Complex | 104 | 63.8 | 72 | 62.6 | 124 | 76.5 |
| Limited Options | 2 | 1.2 | 3 | 2.6 | 11 | 6.8 |
| Duration of Infection | ||||||
| <4 weeks | 3 | 1.8 | 21 | 18.3 | 45 | 27.8 |
| 4–12 weeks | 2 | 1.2 | 11 | 9.6 | 10 | 6.2 |
| >12 weeks | 158 | 96.9 | 83 | 72.2 | 107 | 66 |
Table 3.
Microbiological characteristics.
Table 3.
Microbiological characteristics.
| Characteristic | Osteomyelitis (n (%)) | Fracture-Related Infection (n (%)) | Prosthetic Joint Infection (n, (%)) | p Value |
|---|---|---|---|---|
| Organism classification | ||||
| Staphylococcus aureus | 102 (62.6) | 60 (52.2) | 76 (46.9) | 0.016 |
| Coagulase-negative staphylococcus | 26 (16.0) | 23 (20.0) | 34 (21.0) | 0.479 |
| Streptococcus species | 19 (11.7) | 9 (7.8) | 28 (17.3) | 0.058 |
| Enterococcus species | 10 (6.1) | 11 (9.6) | 14 (8.6) | 0.046 |
| Pseudomonas species | 13 (8.0) | 8 (7) | 8 (4.9) | 0.537 |
| Other Gram negatives | 37 (22.7) | 27 (23.5) | 27 (16.7) | 0.282 |
| Other Gram positives | 28 (17.2) | 15 (13.0) | 13 (8.0) | 0.537 |
| Candida species | 0 | 1 (0.9) | 1 (0.6) | 0.530 |
| Gram-positive organisms only | 118 (72.4) | 77 (67.0) | 126 (77.8) | 0.134 |
| Gram-negative organisms only | 12 (7.4) | 9 (7.8) | 16 (9.9) | 0.694 |
| Polymicrobial infection | 59 (36.2) | 37 (32.2) | 35 (21.6) | 0.013 |
Table 4.
Resistance profiles.
Table 4.
Resistance profiles.
| Osteomyelitis (n (%), 95% CI) | Fracture-Related Infection (n (%), 95% CI) | Prosthetic Joint Infection (n, (%), 95% CI) | p Value | |
|---|---|---|---|---|
| Resistance | ||||
| Confirmed gentamicin resistance | 14 (8.6), 4.3–13.5 | 13 (11.3), 6.1–17.4 | 16 (9.9), 5.6–14.8 | 0.754 |
| Presumed gentamicin resistance | 2 (17.8), 12.3–24.5 | 25 (21.7), 13.9–29.6 | 20 (12.3), 7.4–17.3 | 0.110 |
| Confirmed vancomycin resistance | 4 (2.5), 0.6–4.9 | 3 (2.6), 0–6.1 | 0 | 0.126 |
| Presumed vancomycin resistance | 44 (27.0), 20.2–33.7 | 34 (29.6), 21.7–38.3 | 31 (19.1), 13–25.9 | 0.100 |
| Confirmed gentamicin and vancomycin resistance | 4 (2.5), 0.6–4.9 | 2 (1.7), 0–4.3 | 0 | 0.150 |
| Presumed gentamicin and vancomycin resistance | 18 (11.0), 6.1–16.6 | 13 (11.3), 6.1–17.4 | 17 (10.5), 6.2–15.4 | 0.975 |
Table 5.
Prosthetic material (PJI + FRI) compared to bone infection without implanted metalware (osteomyelitis).
Table 5.
Prosthetic material (PJI + FRI) compared to bone infection without implanted metalware (osteomyelitis).
| Characteristic | Osteomyelitis (n (%)) | Metalwork In Situ (PJI + FRI with Metalwork) | p Value |
|---|---|---|---|
| Organism Classification | |||
| Staphylococcus aureus | 102 (62.6) | 136 (49.1) | 0.006 |
| Coagulase-negative staphylococcus | 26 (16.0) | 57 (20.6) | 0.231 |
| Streptococcus species | 19 (11.7) | 37 (13.4) | 0.605 |
| Enterococcus species | 10 (6.1) | 25 (9.0) | 0.279 |
| Pseudomonas species | 13 (8.0) | 16 (5.8) | 0.369 |
| Other Gram negatives | 37 (22.7) | 54 (19.5) | 0.423 |
| Other Gram positives | 28 (17.2) | 28 (10.1) | 0.537 |
| Candida species | 0 | 2 (0.7) | 0.396 * |
| Polymicrobial infection | 59 (36.2) | 72 (26.0) | 0.024 |
| Confirmed vancomycin + Gentamicin resistance | 4 (2.5) | 2 (0.7) | 0.139 * |
Table 6.
The percentage of each pathogen in prosthetic joint infection.
Table 6.
The percentage of each pathogen in prosthetic joint infection.
| Comparison Site | This Study n = 162 2024, UK | Rupp et al. [27] n = 81 2021, Germany | Triffaut-Fillit et al. [30] n = 567 2019, France |
|---|---|---|---|
| Staphylococcus aureus | 46.9 | 27.9 | 28.9 |
| Coagulase-negative Staphylococci | 21.0 | 23.3 | 28.6 |
| Streptococcus spp. | 17.3 | 10.5 | 13.1 |
| Gram negatives | 21.6 | 10.5 | Not reported |
| Polymicrobial infection | 21.6 | 17.3 | 18.2 |
| Combined gentamicin and vancomycin resistance | 10.5 | 9.9 | Not reported |
Table 7.
The percentage of each pathogen in fracture-related infection.
Table 7.
The percentage of each pathogen in fracture-related infection.
| Comparison Site | This Study n = 115 2024, UK | Patel et al. [26] n = 294 2023, UK | Rupp et al. [27] n = 86 2021, Germany | Depypere et al. [17] n = 191 2022, Belgium |
|---|---|---|---|---|
| Staphylococcus aureus | 52.2 | 24.4 | 37.4 | 31.4 |
| Coagulase-negative Staphylococci | 20 | 14.0 | 16.9 | 25.8 |
| Streptococcus spp. | 7.8 | 4.5 | 7.2 | ‘rarely detected’ |
| Gram negatives | 30.4 | 39.7 | 10.5 | 27.8 |
| Polymicrobial infection | 32.2 | 34.2 | 10.5 | 25.3 |
| Combined gentamicin and vancomycin resistance | 11.3 | 5.8 | 6.8 | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Source link
Annalise Unsworth www.mdpi.com