1. Introduction
The eye, one of the most important sensory systems of an organism, is essential for communication between animals and their environment [
1]. In birds, this sense is highly developed, enabling them to detect small moving objects and identify items in low-light conditions [
2,
3]. Although the basic structure of the eye is consistent among vertebrates [
4], the spatial resolution ability varies significantly [
5,
6,
7,
8,
9,
10,
11,
12]. This variability is linked to the distinct perceptual challenges faced by species in their specific habitats. Differences in visual acuity reflect the adaptations required to overcome these challenges, which vary according to the environment and ecology of each species [
13,
14].
Birds possess a remarkable ability to see in a wide range of light conditions, varying from intense daylight (10
5 lux) to dim starlight (10
−3 lux) [
12]. To adapt to these diverse conditions, they have developed specialized visual characteristics. These include tetrachromatic vision and a retina containing a photoreceptor system composed of five types of cones (four simple cones and one double cone), each sensitive to different wavelengths, and a type of rod [
15,
16,
17]. Pigments in the outer segments of these cone-type photoreceptors allow them to detect visible light, as well as short, medium, and long wavelengths, and in some birds, also ultraviolet light [
9]. Single cones are involved in colour vision, while double cones are likely more associated with processing achromatic signals, texture, and motion [
18,
19,
20,
21].
All cones contain lipid droplets located in the outermost part of the inner segments, each with different carotenoids, so that each type of cone combines an opsin-based visual pigment with a specific type of lipid droplet [
9,
22]. Their main function is to filter incident light before it reaches the outer segments [
23], thereby adjusting the spectral sensitivity of colour vision and enhancing colour discrimination [
24]. Furthermore, recent studies suggest that the impact of oil droplets may vary depending on their transparency and pigmentation, influencing how effectively they modulate light transmission and visual sensitivity [
25,
26]. The distribution of both lipid droplets and photoreceptors has been extensively studied in birds, revealing variations between species, and even among individuals, reflecting differences in visual ecology [
17,
27,
28,
29,
30].
On the other hand, species that require greater visual acuity, particularly for capturing prey, have developed specialized regions in the retina that increase their cone density, most notably a horizontal streak of an increased ganglion cell density with the presence of one or two foveae [
22]. The fovea is an invagination containing a high concentration of densely packed photoreceptors and neurons, providing enhanced visual resolution [
4]. In addition to the existence of these areas of high visual acuity, birds exhibit both quantitative and qualitative variations in the different cell populations that comprise the retina, as well as in the thickness of the different layers, with these adaptations corresponding to the ecological needs of each species.
Birds belonging to the order Charadriiformes represent a globally distributed clade characterized by high ecological and morphological diversity [
31]. This order includes the family Laridae (gulls), seabirds that inhabit a wide range of latitudes and environments, with species nesting in the Arctic, Antarctic, and on tropical coasts. They are also generalist birds in terms of feeding and breeding habits [
32]. The Audouin’s Gull,
Larus audouinii, is a medium-sized gull (average weight of approximately 570 g) endemic to the Mediterranean region [
33]. Although the family Laridae is generally diurnal,
L. audouinii is an ecological outlier in terms of foraging, being one of the few species in the family with a nocturnal foraging pattern. During the night, it takes advantage of the vertical migratory cycles of its prey, feeding mainly on clupeiformes [
34,
35]. This narrow feeding niche is also very rare among gulls and may explain why
L. audouinii has such a restricted distribution [
36]. Therefore, although
L. audouinii has been described as a mostly diurnal species, it exhibits some nocturnal activity [
37].
In this study, we examined the retina of the gull L. audouinii to describe its histological structure and ultrastructure, as well as the morphology of its various constituent cells, and to relate these findings to the ecology of the bird. L. audouinii is a gull species with a lifestyle distinct from other members of the family Laridae. Therefore, if the visual system adapts to the ecological needs of animals, we aim to relate these differences in the lifestyle of L. audouinii to specific visual adaptations present in its retina.
2. Materials and Methods
Experiments were conducted in accordance with European Union and Spanish government regulations and standards. Animals were treated following applicable institutional guidelines for the care and use of animals. A total of three gulls
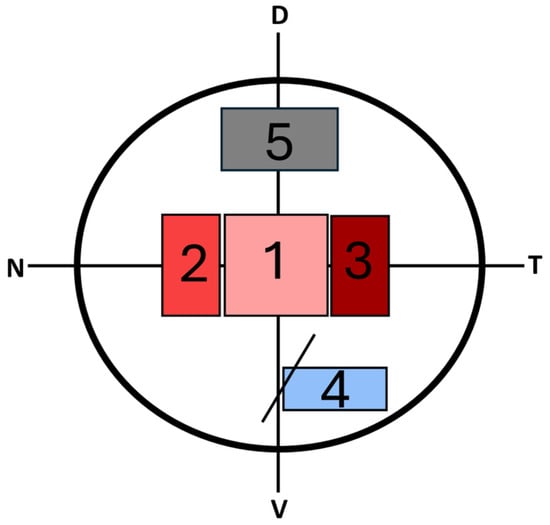
(L. audouinii) collected from the Santa Faz Wildlife Recovery Centre (Alicante) were used. After immobilization, the animals were euthanized via an intravenous overdose of sodium pentobarbital (Eutanax), and their eyeballs were removed. The retinas were sectioned into five regions spanning central to peripheral areas, considering the temporal, nasal, dorsal and ventral zones (
Figure 1). The sections were immersed in a fixative solution (1% paraformaldehyde, 1.6% glutaraldehyde, and 0.15 mM CaCl
2 in a 0.1 M phosphate buffer, pH 7.4) for 2 h and then stored at 4 °C overnight. The next day, the pieces were rinsed in a phosphate buffer and post-fixed in 2% OsO
4 for 1 h. Subsequently, the tissues were dehydrated using a graded ethanol series (70, 90, 96, 100%; 15 min each). The tissues were then cleared in propylene oxide and gradually embedded in Epon-812 epoxy resin. After transferring each tissue piece into a mould, they were incubated at 60 °C for 2 days. Semithin sections (500–1000 nm) were cut, stained with 0.5% toluidine blue, and examined under a Leica DMRB light microscope. Photomicrographs were taken using Lumenera’s INFINITY microscopy camera. Ultrathin sections were double-contrasted with uranyl acetate (0.5%) and lead citrate (0.25%) and examined under a JEOL JEM-1400 Plus transmission electron microscope at 120 kV (Tokyo, Japan) equipped with a Gatan Orius digital camera (Pleasanton, CA, USA) for image acquisition.
3. Results
The retina of L. audouinii exhibits the basic structure typical of vertebrate animals but possesses distinct characteristics, likely due to its adaptation to the environment and its lifestyle.
Among all the regions examined, we considered regions 1 to 3 as the central retina and regions 4 and 5 as peripheral. Region 3 was identified as the area due to its unique features, such as an increased cell density across all cell layers, making the retina thicker than in other regions, as well as the absence of rod photoreceptors.
3.1. Retinal Pigment Epithelium
The retinal pigment epithelium (RPE) of
L. audouinii consists of a single layer of roughly cuboidal cells that contain an abundant number of melanin granules. Basally, the cells of the RPE abut Bruch’s membrane. Apically, the epithelial cells display numerous villi that enclose the photoreceptors’ outer segments (
Figure 2). The RPE exhibited different measurements across the retina: approximately a 20 μm total length in peripheral zones (
Figure 2A) and 32 μm in central zones (
Figure 2B).
3.2. Photoreceptors
The photoreceptor layer is formed by the outer and inner segments of these cells. The retina of
L. audouinii contains rod, single-cone, and double-cone photoreceptors, easily differentiated by the characteristics of their outer segments and by the presence of oil droplets in a cone’s inner segment (
Figure 3).
In the central retina, the photoreceptor layer length varied from 69.92 ± 4.02 μm in region 2 to 76.29 ± 6.42 μm in region 3 (
Figure 4). In these regions (1, 2, and 3) a higher proportion of cones relative to rods was observed, specifically 3:1, 3:1, and 5:1. In the peripheral retina, the photoreceptor layer had a length of 55.72 ± 1.82 μm in region 4 and 67.84 ± 4.22 μm in region 5 (
Figure 4). These regions had a lower proportion of cones compared to rods than in the central retina, with a 1:2 ratio observed in both regions.
3.3. Outer Nuclear Layer
The outer nuclear layer (ONL) contains the cell bodies of photoreceptors, including both rods and cones. Both rod and cone nuclei are arranged in 3–4 rows, whose topographical distribution varies across the retina (
Figure 2). This layer measured approximately 23.8 ± 1.44 μm in the central retina compared to 12.63 ± 2.08 μm in the peripheral retina (
Figure 4).
Using light microscopy, we identified three types of nuclei based on morphological characteristics, whose distribution varies in the different regions (
Figure 5). A group of small, circular nuclei is located close to the scleral region of the ONL. These nuclei occupy the entire cell body and contain very dense chromatin, likely corresponding to the nuclei of rod photoreceptors (N1). One or two rows in the middle part of the ONL contain large, oval nuclei with scarce stained and scattered chromatin (N2). Finally, a row of nuclei deep in the ONL near the OPL contains medium-sized nuclei that are not very dense and occupy the entire cell body (N3). Both N2 and N3 likely correspond to cone photoreceptors.
The proportion of each type of nucleus varies across the retina. The N1:N2:N3 ratio was 2:5:1 in region 1, 5:7:8 in region 2, 5:17:8 in region 3, and 7:5:9 in regions 4 and 5.
3.4. Outer Plexiform Layer
The outer plexiform layer (OPL) (
Figure 2) is formed by the synaptic terminals of the photoreceptors and the extensions of the horizontal and bipolar cells from the innermost layers. In the central region of the retina, its thickness varied between 25.32 ± 2.31 in region 3 and 16.38 ± 1.83 μm in region 1, while in the peripheral area, it had a thickness of 23.25 ± 2.83 μm in region 4 and 21.74 ± 3.33 μm in region 5 (
Figure 4).
3.5. Inner Nuclear Layer
The inner nuclear layer (INL) is formed by the cell bodies and nuclei of the horizontal, amacrine, bipolar, and Müller cells (
Figure 6). Its thickness varied in the central retina from 60.14 ± 5.48 μm in region 3 to 41.27 ± 2.03 μm in region 1, and in the peripheral retina from 24.58 ± 3.64 μm in region 4 to 29.25 ± 7.34 μm in region 5 (
Figure 4).
In the outermost part of this layer, underlying the external plexiform layer, is a row of horizontal cells along the entire retina. Two distinct morphological types of horizontal cells could be identified (
Figure 6): a small cell with dense nuclei that occupies almost the entire cell body (H1) and a larger cell with less dense nuclei (H2). In the most central regions (1, 2, and 3), these two types of horizontal cells had a very similar H1:H2 ratio of 3:2. In the peripheral retina, H2 horizontal cells were much more abundant, with a ratio in both regions of 1:4.
Below this row of horizontal cells, we observed bipolar cells with small, rounded nuclei, forming longitudinal rows that varied in number in each region of the retina. In regions 1 and 2, between 10 and 12 rows were observed, and in region 3, 15–16 rows were observed. In the peripheral retina, they were distributed in fewer rows, between 5 and 6.
Finally, in the innermost zone of the INL, amacrine cells could be observed, which are round to oval and possess an abundant cytoplasm. These cells have a similar morphology and quantity in all regions of the retina, both the central and peripheral zones.
The Müller cells extend radially through the retina and present an irregular cell body located in the middle part of the INL. These cells have a very dark cytoplasm.
3.6. Inner Plexiform Layer
The inner plexiform layer (IPL) contains the processes of bipolar cells, amacrine cells, and ganglion cells. In the central area of the retina, its thickness ranged from 73.71 ± 2.15 μm in region 3 to 55.67 ± 2.11 μm in region 1 (
Figure 4). In the peripheral zone, its thickness was 36.83 ± 6.58 μm in region 4 and 40.88 ± 8.25 μm in region 5 (
Figure 4).
3.7. Ganglion Cell Layer
The ganglion cell layer is generally a single cell thick in the peripheral retina, while it can be two to three cells thick in the central retina (
Figure 2). Ganglion cell perikarya exhibit a voluminous appearance and have numerous dendrites that establish synapses with the bipolar and amacrine cells at the level of the IPL. Their axons are located parallel to the inner surface of the retina, forming the nerve fibre layer. The length of this layer was quite similar in the peripheral and central areas, measuring 11.75 ± 1.35 μm in the central retina and 6.08 ± 0.84 μm in the peripheral retina (
Figure 4). It is important to point out that the peripheral retina has fewer cells than the central retina.
3.8. Optic Nerve Fibre Layer
The thickness of the nerve fibre layer (NFL) varies significantly depending on the part of the retina being examined. Its thickness ranged from 18.21 ± 2.11 μm in region 2 to 6.21 ± 3.2 μm in region 5 (
Figure 4). In region 4, close to the pecten oculi, it reached up to 69.9 ± 7.43 μm.
Finally, the inner limiting layer is formed by the foot-shaped enlargements of the Müller cells, which create a basal lamina that defines the innermost boundary of the retina (
Figure 7).
4. Discussion
Visual ecology focuses on studying how the visual systems of organisms adapt to their environment, optimizing perception to meet essential needs such as survival, feeding, and reproduction. One of the first works to highlight the relationship between the visual system and the environment in which species live was ‘The Vertebrate Eye and Its Adaptive Radiation’ by Walls [
4]. This integrative work, which compiled various studies on the visual system of vertebrates, is considered fundamental for understanding the basic structure of the eye. Subsequently, research such as that of Hughes [
38] demonstrated that there is a close relationship between the lifestyle of organisms and the configuration of their visual system, particularly the retina. Understanding how each aspect of the retina’s design has been optimized over time to meet the visual needs of different species is a fundamental principle in visual ecology [
9,
39].
In this context, the retina of
L. audouinii is an excellent model for visual ecology studies due to its specialized adaptations to both marine and aerial environments. This retina exhibits the same pattern of layer distribution as in other vertebrates but is considerably thicker, comparable to that of other diurnal birds [
40,
41]. This increased thickness is due to a higher cellular density in the nuclear and inner plexiform layers, reflecting the complexity of information processing before it reaches the ganglion cells that transmit it to the brain [
17,
42,
43,
44]. Additionally, the retina is thicker in the centre compared to more peripheral regions, and within the centre areas, a region of high visual acuity has been identified. This enables the gull to detect prey in the high-contrast, brightly lit environments typical of marine habitats, making it a valuable model for studying the relationship between visual adaptations and the ecology and behaviour of seabirds.
4.1. Epithelium Pigment
The structure of the pigment epithelium in
L. audouinii is quite like that described in other vertebrates, consisting of a single layer of cuboidal cells [
45], which connects the choroid with the neural retina [
46].
The pigment epithelial cells in Audouin’s Gull exhibit a morphology characterized by melanin granules concentrated in their apical extensions. These findings align with those described for the pigment epithelium of the Mallard (
Anas platyrhynchos) [
45], the Emu (
Dromaius novaehollandiae) [
47], and even other gulls, such as the Yellow-legged Gull (
Larus michahellis) [
44], the Ring-billed Gull (
Larus delawarensis), and the Grey Gull (
Larus modestus) [
41]. This arrangement of melanosomes corresponds to a diurnal lifestyle, unlike that of nocturnal birds, in which the granules are located at the basal side of the epithelial cells. The integrity of the pigment epithelium is essential for vision and serves important functions, notably light absorption and the protection of the retina against photooxidation [
48], especially the photoreceptors. This protection is provided by melanin granules or melanosomes [
46]. In the case of gulls, such as Audouin’s Gull, this epithelium is crucial. These birds are constantly exposed to light, as there is no shade in their marine habitat. Additionally, some studies have reported nocturnal behaviour in these gulls, particularly for feeding [
49], so these extensive cell extensions may serve a dual role: protecting against excessive light during the day and providing structural support to the outer segments of the photoreceptors, properly orienting them to capture light in the correct direction and thus be more efficient in low-light conditions [
46,
50].
4.2. Photoreceptor Layer
The thickness of the photoreceptor layer varies depending on the region of the retina, being greater in central regions than in peripheral regions. Differences were observed between the peripheral regions 4 and 5; region 5 had greater thickness than region 4, and this may be due to its location in the dorsal region, which is essential for obtaining a clear downward image during the feeding process. It is important to note that this species exhibits nocturnal feeding patterns. As observed in other diurnal birds, Audouin’s Gull exhibits a high proportion of cones compared to rods [
2,
15,
51,
52], since cones function in bright light conditions and are responsible for colour vision. The predominance of cones in diurnal animals allows the retina to respond more rapidly to the light input and to adapt to a wide range of light intensities [
53]. The abundance of cone-type photoreceptors in the retina of the Audouin’s Gull suggests that colour vision and daytime vision are important for this animal’s daily activities [
2,
9].
The proportion of cones to rods in the retina of
L. audouinii varies across different regions. In the central retina, there is a high proportion of cones relative to rods, reaching a maximum value (5:1) in region 3, where most of the observed photoreceptors are cone-type. Additionally, this region shows greater thickness due to its increased cellular density. These features, in combination with previous findings reported in the literature, strongly suggest that this is an area of high visual acuity [
4,
17,
54,
55,
56,
57,
58,
59]. In peripheral regions, the proportion decreases, with the cone-to-rod ratio being 1:2. Nonetheless, the high number of cones throughout the tissue indicates a retina well adapted to daytime conditions. These findings partially coincide with those obtained in other gull species such as
Larus michahellis [
30,
44],
Larus delawarensis, and
Larus modestus [
41]. Regarding rod-type photoreceptors,
L. audouinii does not exhibit a uniform distribution of rods, unlike other species of the same genus, such as the Ring-billed Gull (
Larus delawarensis) and the Grey Gull (
Larus modestus), which have significant nocturnal activity and conduct activities such as feeding and reproduction in the absence of light [
41].
L. audouinii displays occasional nocturnal behaviour, particularly for fishing, so the concentration of rods is not uniform but is slightly increased in the central area, which may be sufficient to support this task at night.
In the ONL, three different types of nuclei were identified: the N1-type nucleus, located in the upper part of the nuclear layer, small in size with very dense chromatin, which corresponds to the nucleus of rod-type photoreceptors, as its morphology matches that described in the Emu (
Dromaius novaehollandiae [
47]), in the Yellow-legged Gull (
Larus michahellis [
44]), and in the Booted Eagle (
Aquila pennata [
17]). On the other hand, N2- and N3-type nuclei belong to cone-type photoreceptors. In region 3, the proportion of N1-type nuclei decreased significantly compared to N2 and N3, which corroborates that this region corresponds to the area of highest visual acuity. This area allows for greater visual acuity, as cone-type photoreceptors have a lower convergence towards bipolar cells and subsequently towards ganglion cells and cells of the visual cortex [
2,
60].
Additionally, the thickness of the outer plexiform layer remains constant throughout the retina of
L. audouinii, with values ranging between 21 and 25 µm. In other species of the genus
Larus, such as
L. delawarensis and
L. modestus, Emond et al. [
41] reported uniformity in this layer; however, in these species, the outer plexiform layer does not exceed 10 µm in thickness. This difference suggests a greater number of bipolar cells and photoreceptors in Audouin’s Gull, which would result in greater visual acuity compared to the other two species.
4.3. Inner Nuclear Layer and Inner Plexiform Layer
In the retinas of diurnal birds, three types of horizontal cells have been described: short-axon horizontal cells (SAHCs), which correspond to the brush-shaped horizontal cells described by Cajal [
40], and two types of axon-less horizontal cells (ALHCs) [
61]. However, in nocturnal birds, such as the Barn Owl (
Tyto alba), and crepuscular species, like the Little Owl (
Athene noctua), only two types of horizontal cells have been reported: SAHCs and ALHCs [
62]. This suggests a possible evolutionary adaptation to low-light conditions, where a simplification in the types of horizontal cells could optimize visual signal integration. Our results show that the Audouin’s Gull presents two types of horizontal cells: a smaller type, whose dense nucleus occupies most of the cell body (H1), and a larger type with a pale cytoplasm (H2). These cells may correspond to the ‘stellate’ and ‘brush-shaped’ types described by Cajal [
40]. The presence of only two types of horizontal cells aligns with the behaviour of Audouin’s Gull, which is not strictly diurnal but exhibits activity during dawn and dusk, when light conditions are variable.
The INL in
L. audouinii is mainly occupied by bipolar cells, organized in rows. In this species, these rows range from 5–6 layers in peripheral regions to 15–16 layers in the central areas. Although Rodieck [
40] described different types of bipolar cells in avian retinas, we could not distinguish these subtypes in our material. In the central region of the retina (region 3), the highest number of bipolar cell rows and the greatest inclination of these cells were observed, suggesting an adaptation for the more efficient processing of visual signals in areas with high visual resolution. Additionally, the IPL is notably thicker in this region, indicative of a high degree of complexity in synaptic interactions and neural processing. This may reflect a functional adaptation that enables
L. audouinii to respond effectively to varying light levels and optimize the capture of visual information in changing environments.
4.4. Ganglion Cell Layer and Optic Nerve Fibre Layer
Ganglion cells receive visual information from photoreceptors via bipolar and amacrine cells [
63]. In the central retina, the degree of convergence between photoreceptors and ganglion cells is low [
64], resulting in a higher number of ganglion cells. This aligns with our findings, with region 3 exhibiting the highest density of ganglion cells. This region is characterized by high visual acuity due to its abundance of cone photoreceptors, thick plexiform and nuclear layers, and numerous ganglion cells.
The low degree of convergence in central regions minimizes the branching of visual information along retinal neurons, enhancing visual acuity. In areas of highest acuity, like the fovea, functional units comprise a cone, a bipolar cell, and a ganglion cell [
64]. As we move peripherally, the convergence increases, with multiple photoreceptors converging onto a single bipolar cell and multiple bipolar cells converging onto a ganglion cell [
64]. This results in fewer ganglion cells in peripheral regions, as observed in the Audouin’s Gull, leading to lower visual acuity.
Ganglion cell axons form the optic nerve, transmitting visual information to the brain. Regions 1, 2, 3, and 5 had similar nerve fibre layer thicknesses. However, region 4 exhibited a significantly thicker layer due to its proximity to the optic nerve head. As suggested by Michaelson [
65] and Prince [
66], the optic nerve converges from the temporal–ventral retina, with the pecten projecting into the vitreous humour. Regions farther from this zone have a thinner fibre layer due to fewer converging ganglion cell axons. As we approach this zone, more axons converge, increasing the layer’s thickness.