1. Introduction
The success of conventional plant breeding relies heavily on the presence of genetic variations within the population of a crop species to facilitate the introgression of the beneficial traits into an elite cultivar [
1]. However, transferring beneficial alleles from genetically diverse backgrounds, such as landraces or wild relatives, into commercial cultivars is time-consuming and often results in linkage drag, introducing unwanted alleles alongside beneficial ones [
2]. Although the introduction of foreign DNA into plant genomes through a transgenic approach has been a focus of crop improvement [
3], the transgenic approach does not harness a plant’s native genetic repertoire to create traits of agricultural value. Therefore, public concerns over the incorporation of transgene from distantly related organisms into crop plants and the governments’ regulatory safety concerns have impeded the widespread use of the transgenic approach for crop improvement [
4].
With the advanced demonstration of the CRISPR/Cas9 system as a tool for crop genome modification, it has become easier to introduce the required genetic variability into any established cultivar directly by targeted genome editing without any requirement of actual mutant lines and the time-consuming plant breeding processes [
5]. The Cas9 introduces double-strand DNA breaks (DSBs) in any defined region of the genome, guided by 20 base gRNA sequences [
6,
7,
8]. These DNA breaks are frequently repaired by the DNA damage repair machinery existing in all eukaryotic systems. The double-stranded DNA breaks are most frequently repaired through non-homologous end-joining (NHEJ) processes, and this process often results in the deletion or addition of a few nucleotides, causing a frameshift and/or gene knockout [
9,
10]. Alternatively, DSBs can be repaired by the homology-directed repair (HDR) mechanism, where a repair template with homologous sequences facilitates precise sequence modification or gene knock-in [
11,
12]. However, the precise selective substitution of nucleotide sequences using HDR-mediated genome editing is more challenging to implement because the cleavage of DNA by Cas9 must be coordinated with the delivery of a DNA repair template and subsequent selection and/or identification of properly edited cell line or plant [
13,
14].
NHEJ is the most common double-strand break repair mechanism in most organisms, including higher plants [
15,
16]. Previously, some success has been reported in enhancing HDR efficiency by suppressing the NHEJ pathway both in vitro and in vivo [
17,
18,
19,
20]. The delivery of a stable and persistent repair template or donor DNA remains a significant hurdle in achieving efficient HDR in higher plants [
21,
22,
23]. Moreover, the delivery of genome editing components through
Agrobacterium-mediated transformation into plant tissue is not very efficient because the transformed repair template, as a part of the T-DNA region, often integrates into the plant genome, making it unavailable for HDR-mediated DNA repair. The screening and identification of successfully knock-in edited cell lines and the subsequent regeneration of a viable plant is another difficulty. These factors make it difficult to successfully utilize HDR-mediated genome editing in plant systems. Despite these difficulties, HDR-mediated genome editing has the higher potential to introduce precise, user-defined genetic variabilities, facilitating targeted trait manipulation in crop plants.
To address these critical rate-limiting factors associated with gene knock-in in plants, we developed an autonomously replicating wheat dwarf virus (WDV)-derived delivery system to enhance HDR efficiency for precision crop improvement. We targeted the rice gene encoding for EPSP synthase for selective amino acid substitution to create a rice plant resistant to the broad-spectrum systemic herbicide glyphosate. Glyphosate is a strong inhibitor of EPSP synthase, a critical plant enzyme involved in the biosynthesis of aromatic amino acids. The availability of sound knowledge about the mode of molecular inhibitory interaction of this herbicide, and the availability of genetic and molecular information regarding the herbicide-resistant mechanism suggests the desired sequence modifications to edit for herbicide tolerance in any given crop plant. The double amino acid substitution (T173I and P177S) mutant EPSP synthase confers significant resistance to glyphosate [
24]. These amino acid substitutions in rice EPSP synthase are expected to reduce or destroy the ability of the herbicide glyphosate to interact with the (T173I and P177S) mutant EPSP synthase, while simultaneously retaining the capacity for normal functioning in the presence of herbicide.
3. Discussion
In this study, we achieved targeted amino acid substitutions (T173I and P177S, or TIPS) within the native
EPSP synthase gene in rice (
Oryza sativa ssp. Indica cv MTU1010) using a CRISPR/Cas9-mediated HDR approach. Our findings build upon previous work demonstrating that transgenic overexpression of
OsEPSPS with TIPS substitutions retains enzymatic function in the presence of glyphosate [
24].
In our study, we first generated stable
rcoSpCas9-expressing rice lines under a strong constitutive
Zea mayze Ubiquitin promoter (ZmUbq_P), providing a reliable source of Cas9 protein for subsequent editing. Previous studies have shown that constitutive Cas9 expression increases the likelihood of achieving the desired edits in plant systems [
25,
26,
27]. In this work, our Cas9-expressing lines (E1, E2, and E20) were instrumental in enabling HDR-mediated editing when combined with a deconstructed wheat dwarf virus (dWDV) replicon system. Moreover, HDR-mediated editing in plants is often hindered by the dominance of NHEJ repair pathways and the transient availability of repair templates [
28]. In our study, we addressed these challenges by using a dWDV-derived replicon system, which provided a stable source of the repair template synchronized with Cas9-induced double-strand breaks (DSBs). Our results align with findings from other studies using Gemini virus replicons to improve HDR efficiency in plant species [
27,
29,
30]. The circular, autonomously replicating dWDV system allowed us to overcome the limitations of
Agrobacterium-mediated transformation, which often results in stable integration of T-DNA, thereby limiting template availability for HDR [
31]. Our PCR amplification of a 646 bp fragment confirmed the formation of a circular, autonomously replicating dWDV replicon in a transformed callus, and sequencing analysis validated its structure, ensuring that the repair template was available in the correct form for efficient HDR.
The successful introduction of T173I and P177S substitutions in the
EPSP synthase gene of rice provides effective glyphosate tolerance without compromising agronomic performance. Glyphosate is a widely used herbicide that inhibits EPSP synthase in the shikimate pathway, essential for aromatic amino acid synthesis in plants [
32,
33]. The precise editing achieved in this study allows for herbicide tolerance in rice without foreign DNA integration, aligning with other studies that used endogenous gene editing to confer herbicide resistance in crops [
34,
35,
36,
37]. This approach not only enhances crop resilience but also will be useful in addressing regulatory concerns regarding transgene incorporation in crops. One of the advantages of our dWDV-based HDR approach is its potential to create non-transgenic, precisely edited plants. As regulatory frameworks evolve to distinguish between genome-edited and transgenic crops, genome editing without foreign DNA integration could expedite regulatory approval and improve public acceptance [
38,
39,
40]. By avoiding stable T-DNA integration and employing a replicon-based repair template, our approach aligns with recent advancements in Latin America, where countries like Argentina lead in the adoption and field trials of genome-edited crops for research and propagation [
41,
42].
Moreover, the dWDV replicon system has broader applications for precision breeding, particularly in traits where exact genetic modifications are essential. Gemini virus replicons have shown success in enhancing HDR across a range of crops, including monocots and dicots like rice, wheat, maize, citrus, and grape [
28,
43,
44,
45,
46]. By enabling targeted editing of endogenous genes without foreign DNA, the replicon system is well-suited for advancing complex trait engineering and precision breeding. Future studies could optimize this system further by integrating multiplexed gRNA constructs for simultaneous edits, making it an adaptable platform for high-efficiency genome editing in agriculture [
20,
47].
4. Materials and Methods
4.1. Plant Materials, Transformation and Growth Conditions
Seeds of
Oryza sativa L ssp. Indica cv. MTU1010 (Cotton Dora Sannalu), an elite, high-yielding variety with long, slender grains that is derived from a cross between Krishnaveni and IR-64 (year of released 2000) (
https://drdpat.bih.nic.in), were obtained from the Andhra Pradesh Rice Research Institute (APRRI), Maruteru, India. Seeds of MTU1010 were used for two consecutive transformation events: the first with a rice codon-optimized
Streptococcus pyrogens Cas9 (
rcoSpCas9) overexpression cassette, and the second with a deconstructed wheat dwarf virus (dWDV) delivery cassette. The transformation with the
rcoSpCas9 overexpression cassette was selected with 50 mg/L hygromycin concentration and calli from the second transformation event were selected using 25 µmol/L glyphosate concentration. Plant transformation was carried out with Dr. M.K. Reddy’s lab-modified protocol (unpublished). Regenerated seedlings from the transformations were transplanted into earthen pots covered with perforated polybags for hardening (at least for 3–5 days) inside the greenhouse conditions (ICGEB, New Delhi). All the positive plants, along with the control MTU1010 plants, were grown on soil under greenhouse conditions at 26 °C with a 14 h light/10 h dark cycle and a relative humidity of 60–70%. All the necessary fertilizers were applied to nourish the plants routinely and regularly irrigated to ensure proper growth. Healthy plant tissues were harvested and immediately frozen in liquid nitrogen prior to DNA and RNA isolation.
4.2. Preparation of the rcoSpCas9 Expression Cassette
A rice codon-optimized
Streptococcus pyogenes Cas9 (
rcoSpCas9) was synthesized from GenScript (
https://www.genscript.com). The sequence was initially cloned into Entry Clone 1 (-Amp, +Gen selection modified in Dr. MK Reddy’s Lab) with
NcoI and
NotI restriction sites. The
rcoSpCas9 was placed under the control of a constitutively expressing maize Ubiquitin promoter (ZmUbqP) (~1.99 kb, amplified using primers ZmUbq F/ZmUbq R) and a
Nopaline synthase gene terminator (NosT) (~250 bp, amplified using primers nosT F/nosT R). We used
KpnI and
NcoI restriction enzymes to clone the ZmUbq promoter and
NotI and
SacI restriction enzymes for NosT, respectively. The resulting Entry vector 1 (EV1) harboring the
rcoSpCas9 expression cassette (ZmUbqP::rcoSpCas9:NosT) was then transferred into the pMDC99 binary vector, which harbored the
Hygromycin phosphotransferase (
hptII) as a selection marker, using the gateway cloning method. A single round of LR recombination was employed to insert the full
rcoSpCas9 expression cassette (ZmUbiP:rcoSpCas9:NosT) between the left (LB) and right borders (RB) of the pMDC99 backbone (
Figure S1). The final expression vector was transferred into the
Agrobacterium strain (EHA105) by electroporation for rice plant transformation.
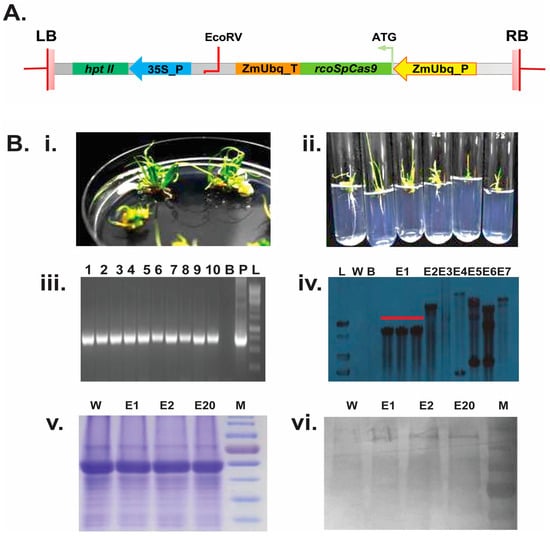
4.3. PCR Screening and Validation of rcoSpCas9 Overexpression Lines
To identify T0-generation positive
rcoSpCas9 overexpressed lines, 35 regenerated plants were used for PCR screening using two sets of primers: hygromycin (hptII F/hptII R) and the junction primer of the maize ubiquitin promoter and Cas9 CDS (Zm JN F/Cas9 JN R) (
Table S1). To determine the copy number of transgene integration, stably transformed T1-generation seedlings were further analyzed using southern blotting, following a lab-modified protocol [
24].
EcoRV restriction enzyme was used to digest the T-DNA segment for southern blot analysis. Souhtern-positive homozygous lines (E2, E10, and E20) were further used for western blot analysis using the Cas9 antibody. The Cas9 antibody was generated following a standard protocol at the ICGEB facility [
48]. For antibody generation, we cloned
SpCas9 in the pET28a expression vector and expressed it in BL21
E. coli cells (
Figure S2).
4.4. Designing and Cloning of Guide RNA and Repair Template
We used the CRISPRdirect (Yuki Naito, 2015) (
https://crispr.dbcls.jp) online software for searching guide RNA target sites in the Indica rice genome, keeping all parameters as default. Two guide RNAs were designed to target the native copy of the rice
EPSP Synthase gene for the substitution of Threonine to Isoleucine at position 173 (T173I) and Proline to Serine at position 177 (P177S) amino acids (together TIPS). These sites are present at the second exon, near the junction of the exon–intron site. Two 20 bp oligonucleotides (gTIPS F and gTIPS R,
Table S1) sequences were selected at the genomic location of 1327 to 1345 bp of
EPSP synthase gene from the position of start codon ATG. To drive the transcription of our synthesized guide RNAs (gTIPS), we used a rice-specific U6 promoter (OsU6_P) and a pol III terminator site at the end of the gRNA scaffold. To multiplex our guide RNA cloning system for future use, we synthesized the entire OsU6 promoter with an arbitrary sequence along with a guide RNA scaffold and pol III terminator from GeneScript under
KpnI and
SacI restriction sites. We incorporated 5′-CAGG-3′ in forward and 5′-AAAC-3′ at the reverse end of the arbitrary sequence to facilitate digestion with
BSaI restriction enzyme and maintained similar overhangs for cloning 20 bp guide RNA, gTIPS. This synthesized construct was cloned into entry clone 1 (EV1, Gen
+).
Further, we designed a repair template of 706 bp lengths with the substitution of T173I and P177S amino acids of the EPSP synthase gene. We also introduced several synonymous nucleotide substitutions without changing the amino acid residues at the target site to prevent Cas9 from re-targeting the donor fragment. Additionally, a unique HindIII restriction site was included in the repair template as a marker for validation in the edited lines.
4.5. Construction of Autonomously Replicating Deconstructed Wheat Dwarf Virus (dWDV)-Based Vector
We developed a binary expression cassette using a multi-round gateway cloning strategy to combine the expression cassette of OsU6 promoter::gRNA (gTIPS):: RNA pol III terminator with the repair template (repTIPS) under the control of autonomously replicating deconstructed wheat dwarf virus (dWDV) system for simultaneous delivery of guide RNA and repair template in the second transformation. The dWDV replicon system was designed with all the necessary components intact which are required for the autonomous viral replication i.e., small intergenic repeats (SIRs) and large intergenic repeats (LIRs). For the possibility of future multiplexing, we also introduced a multiple cloning site (MCS) consisting of restriction enzymes XhoI, NheI, XbaI, NotI, BglII, and SalI sites. The system was first cloned into Entry clone 2 (EV2, Gen+, Chl+) under the EcoRI and SacI restriction enzymes. This EV2-dWDV construct was later used for the cloning of our synthesized repair template (repTIPS), by replacing the WDV coat protein (CP) of dWDV using the XhoI and XbaII restriction sites. Later, multi-round gateway cloning was carried out to in vitro pyramiding the guide RNA construct and repair template into the modified pMDC99 (-hptII) vector backbone.
4.6. Mutation Identification and Inheritance Analysis
Two sets of PCR primers (
Table S1) were used for the amplification of the target region. The final PCR product or ~750 bp fragment was further used for the sequence analysis with the help of the Macrogen sequencing platform (
https://www.macrogen.com). Seeds from the T0-edited lines were used for the mutation inheritance analysis via both germination assay (MS basal media with 50 µmol/L glyphosate) and foliar spray experiments with the herbicide Roundup (active ingredient: isopropylamine salt of glyphosate, 41.0% (
w/
v) with the doze of 2 mL/L). A foliar spray experiment was conducted at the initial tillering stage of the seedlings. The TIPS-edited plants along with the control WT MTU1010 plants were uniformly sprayed with commercial Roundup herbicide and maintained in a greenhouse at 26 °C controlled temperature, 14 h light/10 h dark cycles, and a relative humidity of 60–70%. The survivability was assessed one week after spraying. Control plants sprayed with water were used as a mock control.
4.7. Statistical Analysis
Statistical analyses were performed using one-way ANOVA, and the differences between means were compared using Tukey’s HSD test (p < 0.5) for the relevant datasets.
5. Conclusions
In summary, we successfully established a viral autonomous replicon system to deliver a donor template suitable for synchronizing double-strand DNA breaks through homology-based repair to achieve precise gene knock-in. In this study, we generated the TIPS mutation in the native EPSP synthase gene; these selective T173I and P177S substitutions disrupt glyphosate interaction with the EPSPS enzyme. The genetic engineering of herbicide-resistant rice plants provides a valuable approach to enable the use of environmentally safe herbicides with flexible application time during the entire crop-growing season, ensuring effective weed control. Moreover, the dWDV-based viral replicon system generated in this study demonstrates significant potential for delivering gene-editing components, thereby would be helpful to accelerate the process of precision breeding and advancing crop improvement strategies.