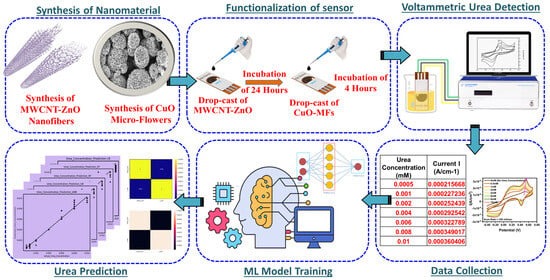
1. Introduction
Urea is an important biomarker in medical diagnostics as it plays a vital role in body detoxifying. It is important for reducing the harmful effects of increased nitrogen levels by altering toxic ammonium ions into urea, which the kidneys then safely excrete through urine [
1,
2,
3]. Hence, urea concentration monitoring is essential for the diagnosis of liver and kidney disorders. Severe conditions such as hyperuricemia, renal abnormalities, acute renal injury, chronic renal disease, nutritional inadequacies, and heart failure can all be indicated by elevated urea levels. Normal blood urea levels range from 1.67 to 7.5 mM, while 342 ± 67 mM is the usual level in 490–2690 mL of urine [
4,
5,
6,
7]. Thus, reliable and precise urea detection techniques are essential for clinical diagnosis as well as for preserving overall wellness.
In continuation, the accurate detection of urea ranges beyond medical diagnostics to various fields, which include food safety, agriculture, and cosmetics. Large-scale urea synthesis has changed the manufacture of nitrogen-based fertilizers in agriculture and has had a substantial environmental impact [
8,
9,
10,
11]. On the other hand, in the food industry, keeping an eye on urea levels is essential for ensuring food safety, especially with dairy products. Sometimes, urea is added to diluted milk to keep it viscous; this needs to be well-monitored to avoid adulteration [
12,
13]. A range of well-established analytical methods, such as infrared spectroscopy [
14], high-performance liquid chromatography (HPLC) [
15], nuclear magnetic resonance (NMR) [
6], calorimetry [
16], fluorimetry [
17], and electrochemiluminescence [
18,
19,
20,
21,
22], have been used to evaluate urea in real blood samples. Although these techniques yield precise results, they have limitations, including lengthy analysis times, expensive equipment, the requirement for trained operators, and labor-intensive specimen preparation. Blood urea nitrogen analysis is the most common technique for measuring blood urea levels to evaluate azotemia [
23,
24]. It is frequently carried out in conjunction with serum creatinine assays. However, because of their complexity and resource needs, these traditional methods are not always feasible for quick on-site testing [
25].
Alternately, electrochemical sensing approaches provide simpler, more cost-effective, and efficient methods to detect urea in blood. These techniques can be used in the food industry, medical field, military, and study of plant biology, among other fields [
26,
27,
28,
29,
30,
31,
32]. Recently, screen-printed electrode (SPE)-based electrochemical biosensors have gained attention for their potential in rapid, sensitive, portable, cost-effective, and precise investigations. Moreover, screen-printing has been suggested as a mass-producible, inexpensive, dependable, single-use sensor technique for on-site monitoring for the past thirty years. SPEs allow the coupling of many carbon-based electrodes with functionalized compounds in an inexpensive, repeatable, and disposable arrangement. When combined with SPEs, electrochemical biosensors can provide a practical substitute for conventional analytical methods for in-field screening and monitoring. Generally, SPEs consist of an electrochemical cell printed on a solid substrate with three electrodes: the reference electrode (RE), counter electrode (CE), and working electrode (WE). These biosensors fall under the following categories: impedimetric, voltammetry, amperometric, and potentiometric [
27,
33]. When using enzymatic or non-enzymatic techniques for electrochemical detection, urea is found by monitoring redox reactions [
23,
24,
34,
35].
The selection of working electrode materials is a critical step in the development of efficient electrochemical sensing platforms. This choice directly affects sensor performance attributes such as low cost, high electrocatalysis, sensitivity, selectivity, stability, electrical conductivity, and biocompatibility. Numerous nanostructured materials, including nanopores, nanoparticles, nanofibers, nanowires, and nanotubes, have been thoroughly studied by researchers [
36,
37,
38]. Carbon-based materials, including graphene, reduced graphene oxide, and carbon nanotubes, as well as metal oxide nanostructures such as zinc oxide, nickel oxide, manganese dioxide, and copper oxide, have attracted considerable interest because of their distinct electrochemical, catalytic, and electrical characteristics. These materials can be accurately altered in terms of their physical structure and surface properties, which is crucial for improving the performance of sensors. Graphene and its derivatives, renowned for their exceptional electrical conductivity and expansive surface area, are especially well-suited for sensors that necessitate fast electron transmission. Moreover, metal oxide nanostructures provide customized chemical reactivity and stability, which are crucial for detecting certain analytes. By adjusting the structure and surface characteristics of these materials, it becomes possible to tailor them according to the unique needs of sensors. This leads to enhancements in sensitivity, selectivity, and overall sensor performance. The capacity to adapt and regulate their properties makes carbon-based and metal oxide materials essential for the development of sensor technologies in environmental monitoring, biomedical diagnostics, and industrial process control applications [
39,
40,
41,
42,
43]. Upon combining them with electrochemical electrodes and with enhanced conductivity, catalytic activity, and binding affinity for target biomolecules [
43], these characteristics enhance the detection signal and make it easier for analytes to react on electrodes enhanced with metallic nanoparticles. As an alternative, attaching ZnO NPs to the MWCNT surface causes the network to form nanotube (NT) and nanoparticle (NP) combinations, which substantially varies the electrical conductivity [
32].
In enzymatic urea sensors, the urease enzyme combined with various metal oxide nanocomposite materials has shown potential for urea detection; however, practical applications are limited by issues such as weak conductivity, a narrow detection range, and high urea detection thresholds. These difficulties highlight the continuous attempts to increase metal oxide nanostructures’ effectiveness and expand their range of applications in sensing technologies. Several innovative biosensors for urea detection have been developed using various advanced techniques and materials [
44,
45,
46,
47,
48]. Enzymatic urea sensors encounter challenges such as enzyme immobilization difficulties, high costs, reproducibility issues, and limitations in operational parameters such as temperature, pH, and humidity [
49]. Consequently, non-enzymatic electrochemical biosensors have been explored for urea detection. In this approach, urea undergoes oxidation/reduction on suitable electrodes [
50,
51]. Researchers innovatively developed an electrochemical sensor using a composite of MWCNT, SWCNT, graphene, and polyaniline (PANi) without enzymatic involvement. The synthesis involved grafting PANi onto graphene through CV, which was validated using Raman spectroscopy. This sensor exhibited enhanced sensitivity and a reduced detection limit, as well as demonstrated outstanding reproducibility, specificity, and durability. It effectively quantified urea levels in both water and milk samples [
49]. This advancement offers a straightforward and cost-effective approach applicable to clinical diagnostics, milk quality assessment, pesticide production, and environmental monitoring for pollutants.
A new biosensor for urea detection was created using a porous composite catalyst composed of nickel-metal organic Framework (Ni–MOF) and MWCNTs. The electrode, fabricated on ITO glass, exhibited strong performance in detecting urea, boasting a high sensitivity of 685 μAmM
−1 cm
−2 and a rapid response time of just 10 s. The biosensor achieved a LoD of 3 μM and demonstrated stability over a storage period of 30 days. The combination of Ni–MOF and MWCNTs in the electrode design leverages their synergistic effects, significantly enhancing the electrocatalytic activity for both urea oxidation and reduction reactions [
52]. Similarly, a glossy carbon electrode (GCE) incorporating silver-doped single-walled carbon nanotubes (SWCNTs) was developed using a simplified thermal reduction process. This electrode exhibited a linear range from 66.0 nM to 20.6 mM for urea detection, with a sensitivity of 141.0 μAmM
−1 cm
−1 and a LoD of 4.70 nM. The electrode’s performance was evaluated in practical scenarios, successfully measuring urea levels in tap water and dairy milk [
53]. These advancements highlight the potential of composite catalysts and nanomaterials in developing efficient biosensing platforms for urea detection, with implications for various applications, including environmental monitoring and food quality assessment.
An ultrathin Ni-MOF nanobelt sensor showed superior efficiency with a linear range of 0.01–7.0 mM with LoD of 2.23 μM and sensitivity of 118.77 μA mM
−1 cm
−2 for urea in biological and environmental samples [
54]. A GCE modified with nickel cobalt oxide (NiCo
2O
4) nanoneedles, synthesized via a low-temperature aqueous method, was developed for non-enzymatic urea detection. This sensor offers a linear response R
2 = 0.99 over 0.01–5 mM and a LoD of 1.0 µM. It overcomes NiO and Co
3O
4 nanoparticles’ poor conductivity, providing a cost-effective, highly selective urea estimation tool [
55]. An Ag/NiOOH nanorods-modified electrode was developed for non-enzymatic urea detection, operating effectively in neutral pH. It shows a higher sensitivity of 233.7 μAmM
−1 cm
−2 over a linear range of 0.2–26.0 mM, with a quick response time of ~3.0 s and a LoD of 5.0 μM in neutral phosphate-buffered saline [
56].
Table 1 shows the MWCNT-ZnO/CuO-MFs modified non-enzymatic biosensors with earlier reported biosensors for urea detection and various electrode materials used, emphasizing the critical role of nanomaterials in enhancing sensor performance. Sensitivity, limit of detection (LOD), and the linear range are the primary factors influencing the effectiveness of these sensors. Among the materials, Ag-N-SWCNTs exhibit the lowest LOD (4.7 nM) and impressive sensitivity (141 μAmM
−1 cm
−2), making them highly effective for detecting even minute concentrations of urea over a wide linear range (66 nM to 20.6 mM). Carbon nanotubes (CNTs), both single-walled (SWCNTs) and multi-walled (MWCNTs), are widely used because of their high electrical conductivity and surface area. For instance, Ni-MOF/MWCNTs show a balance of high sensitivity (685 μAmM
−1 cm
−2) and low LOD (3 μM). The use of metal oxides such as NiO and CuO combined with CNTs further improves sensor efficiency. The integration of machine learning (ML) in the MWCNT-ZnO/CuO-MFs electrode underscores the future potential of using ML algorithms for sensor optimization. This sensor achieves high sensitivity (117.98 mA mM
−1 cm
−2) and a very low LOD (78.479 nM), highlighting how ML can assist in better calibration and data processing.
The integration of machine learning (ML) and electrochemical sensing is becoming an innovative approach that offers an unmatched ability to decode complex data patterns. Large-scale electrochemical data sets can be evaluated by ML algorithms, which can also identify minor correlations and trends that conventional methods might miss. This results in more accurate measurements that address important urea detection difficulties such as selectivity, specificity, and sensitivity. Moreover, ML has the capacity to adjust and enhance sensor performance over time, guaranteeing reliable and superior results. In addition to improving urea detection, the combination of ML and electrochemical sensing opens new possibilities for quick, precise, and scalable biosensing applications. This novel approach shows promise in a variety of sectors, including clinical diagnostics, environmental monitoring, and industrial process control, transforming how we detect and analyze biological compounds [
63,
64,
65].
This study aims to pioneer the development of an ML-assisted, flexible, electrochemical, non-enzymatic biosensor for precise urea concentration detection. Leveraging a novel MWCNT-ZnO nanocomposite functionalized with CuO-MFs, we seek to enhance sensitivity and operational performance, addressing key challenges faced by traditional biosensors. Our comprehensive evaluation incorporates various characterization techniques and electrochemical CV analysis to validate the biosensor’s effectiveness. Furthermore, by integrating advanced ML models to predict urea concentrations from experimental data, we enhance the sensor’s accuracy and reliability. This research not only demonstrates the transformative potential of ML in sensor technology but also paves the way for innovative applications in clinical diagnostics, environmental monitoring, and food safety. The findings of this study hold significant promise for advancing biochemical sensing technologies, ultimately contributing to improved health outcomes and more effective monitoring of urea levels in diverse settings.