1. Introduction
Under the conditions of a long space flight, humans will inevitably be exposed to high levels of chronic radiation, which are dangerous to life and health. Up-to-date defense protection engineering systems are not sufficient to fully address and provide comprehensive health care for astronauts. Pharmaceutical administration attempts to improve the human defense system through the use of radioprotectors [
1,
2] and natural substances derived from plants and other biological sources [
3]; however, these techniques have limited effectiveness [
4]. Several Food and Drug Administration (FDA)-approved drugs, including statins, nonsteroidal anti-inflammatory drugs, and angiotensin-converting enzyme inhibitors, show potential as radiation countermeasures [
5]. Amifostine is currently the only FDA-approved agent for the treatment of high-dose radiation exposure, with a dose reduction factor of up to 2.4 [
6]. However, its effectiveness for chronic low-dose space radiation is uncertain. For this reason, the idea of creating long-term biological protection using natural mechanisms of radioprotection, which, unlike existing low-molecular compounds, provide comprehensive protection of cells of an organism from different types of radiation, has emerged [
7]. Current methods of gene therapy for radioresistance are based on the endogenous expression of the desired genes delivered to the cells via plasmid liposomes and adenoviral systems [
8], but this approach and other methods associated with direct modification of human cells may not be safe. In light of the fact that the genetic modification of human beings remains questionable from both a medical and an ethical point of view [
9], indirect approaches to gene editing are needed, probably based on the modification of the resident microbiota.
We take a look at the opportunities of bioengineered probiotics which could potentially enhance the radioresistance of host cells through their secretory activity or interactions with intestinal mucosal cells. Indeed, there are some cases of probiotics with similar effects, for example,
Lactobacillus rhamnosus GG, which has been shown to modulate immune responses and protect intestinal cells from radiation-induced damage [
10].
Bifidobacterium breve is known for its ability to enhance the mucosal barrier function and reduce inflammation [
11]. Another example is
Escherichia coli Nissle 1917, which has demonstrated protective effects against oxidative stress in the gut [
12]. The use of gene-engineered probiotics could lead to targeted effects by enabling the precise delivery of therapeutic molecules or the modulation of specific cellular pathways. This approach holds promise for enhancing the resilience of bowel cells to radiation, potentially benefiting patients undergoing radiotherapy by reducing gastrointestinal side effects. Furthermore, the bioengineered probiotics could be tailored to produce specific enzymes or antioxidants that neutralize reactive oxygen species, thereby mitigating DNA damage and promoting cellular repair mechanisms [
13]. Radiation exposure can disrupt the gastrointestinal system by damaging stem cells and altering signaling pathways crucial for intestinal homeostasis [
14]. The use of radioprotectors may facilitate the stabilization of cell membranes and the reduction in permeability to harmful agents, thereby assisting in the maintenance of cellular structure under conditions of stress. For instance, probiotics have demonstrated the capacity to safeguard mucosal integrity and to diminish oxidative stress within the intestine and brain [
15]. Following absorption in the intestine, radioprotectants enter the bloodstream, thereby enabling their circulation throughout the body. This enables them to reach various organs and tissues, thereby providing protection against radiation-induced damage at the systemic level. The integration of synthetic biology with probiotic therapy could revolutionize the management of radiation-induced gastrointestinal toxicity, offering a novel and personalized strategy for patient care [
16].
The aim of our study was to discuss the advances in the microbiota-mediated approach to enhance human resistance to ionizing radiation.
3. Microbiome-Induced Space Suit
In the original form, our project entitled “Microbiome-Induced Space Suit” (MISS) considers the development of several types of genetic constructs under control by an arabinose-inducible promoter pBAD [
1]. This means that the expression of radioprotective proteins will only be activated under the influence of arabinose, which can be administered orally if necessary, thus minimizing side effects. The effector part of the construct contained multiple sequences encoding radioprotective proteins and regulatory microRNAs. We used two approaches to identify the most prominent targets. First, we analyzed information on the transcriptome of people living in regions with a high natural radiation background. It has been shown that people who live in such areas for a long time do not have an increase in the number of diseases associated with radiation exposure compared to people who are not chronically exposed to radiation [
28]. This means that humans have a natural defense mechanism against radiation that could include enhanced DNA repair processes, efficient cellular stress responses, or other protective biochemical pathways. Based on this, one of the ideas of the MISS project is to identify the specific molecular mechanisms that allow humans to tolerate high levels of radiation exposure without adverse health effects. This approach involves searching for the expression of unique genes, regulatory pathways, and protein functions that are regulated in these populations. Once the relevant molecular mechanisms are identified, genetic constructs can be designed and used to modify the human microbiome and recreate the protective effects observed in populations exposed to high levels of radiation.
The second approach is to use human proteins that are homologous to the tardigrade protein DNA damage suppressor protein (DSUP), allowing them to exist at very high levels of radiation lethal to humans [
29]. As a result, we used the well-studied proteins Wnt family member 10A (Wnt10a) and Bloom’s syndrome helicase (BLM). Wnt10a plays a crucial role in wound healing and tissue regeneration by regulating collagen expression and synthesis [
30]. Its absence leads to delayed wound healing and reduced fibroblast/myofibroblast numbers [
31]. BLM plays an important role in DNA damage repair and genome stability by functioning as part of the BLM dissolvasome complex, which resolves bound DNA intermediates without genetic exchange [
32]. Both of these proteins are involved in cellular stress resistance (
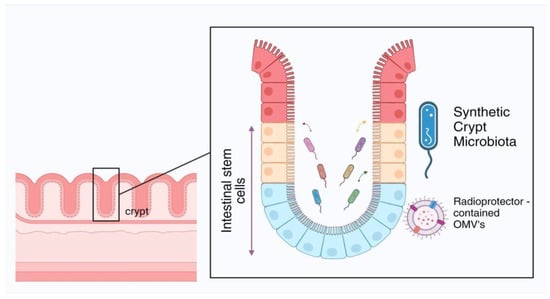
Figure 2A). The proposed pathway of OMVs-mediated radioprotector delivery into intestine crypt cells through gene-engineered microbiota is presented in
Figure 2B. The key challenge in the implementation of this approach is the direct transport of nanoparticles through mucin media to the bacterial cells (distance ~100–200 μm), as well as the OMV transport to host cells at a distance of 20–50 μm [
33].
The proposed idea of the MISS project is that two plasmids that encode the target genes for the radioprotective molecules (BLM and Wnt10a) are conjugated with silicon nanoparticles by chemical or biological methods. Subsequently, the nanoparticles are orally administered into the intestine and may be able to transform the luminal and crypt-specific microbiota. Once the plasmids are integrated into the bacterial genome, these bacteria begin to express new genes, resulting in the formation of OMVs containing radioprotective proteins and RNA. The OMVs then fuse with the intestinal crypt cells, resulting in the release of their cargo into the cytoplasm of the host cell, with efficacy depending only on the radioprotective properties of the defense genes used.
The spectrum of radioprotective defense genes may extend far beyond the currently well-known pathways. Recently, it has been found that the induction of radiotolerance in human cells could be achieved by adopting information transfer from tardigrade cells [
34]. Tardigrades, known for their remarkable resilience to extreme environmental conditions, provide a unique model for understanding the mechanisms of radiation tolerance. By studying the molecular pathways and protective proteins in tardigrades, researchers aim to develop strategies to enhance the resistance of human cells to radiation [
35,
36]. This issue also examines the cases of successful adaptation to radiation in humans and the challenges faced in developing comprehensive therapies to induce cellular radioresistance [
37], a critical aspect for space exploration [
7], and also explores using high-altitude isolated biological reservoirs as potential sources of promising radioprotectors [
38]. These exotic reservoirs, often found in extreme environments, harbor organisms that have naturally evolved mechanisms to withstand high levels of radiation [
39]. Tapping into these biological resources holds promise for discovering novel compounds or genes that can be used to protect human cells from radiation-induced damage.
Microbiome studies provide insight into the maintenance of gut health with probiotic consortium supplementation and will facilitate the development of probiotic-based therapeutic strategies for radiation-induced gut injury. Some types of secretory activity of gut microbiota showed the radioprotective effect on intestine cells. Espinal et al. showed the abilities of oral administration of second-generation probiotic
Lactobacillus-reuteri-Interleukin-22 is an effective protector and mitigator of intestinal irradiation damage, by improving the capacity of therapeutics to stabilize the number of Lgr5+ intestinal crypt stem cells and their progeny [
40]. In a mouse model, it has been shown that intestinal microflora (
Lachnospiraceae and
Enterococcaceae) and its production of short-chain fatty acids and specific tryptophan metabolites can tune host resistance against high doses of radiation from facilitating hematopoiesis and gastrointestinal recovery [
41,
42]. Fasting-induced adipose factor (angiopoietin-like 4, ANGPTL4) is a microbiota-regulated, epithelial-derived, secreted protein participating in radioresistance and suggested to be useful as a gut radioprotector [
43,
44]. The probiotic consortium mixture of
Bifidobacterium longum BL21,
Lactobacillus paracasei LC86, and
Lactobacillus plantarum Lp90 attenuated radiation-induced intestinal injury by modulating the gut microbiota and metabolites, improving inflammatory symptoms, and regulating oxidative stress [
45].
Advances in the genetic engineering of the gut microbiota, including transient expression systems, represent a promising frontier in the development of next-generation probiotics. These innovative approaches aim to enhance the beneficial properties of gut bacteria, enabling more precise and effective interventions to promote human health. Specific genetic modifications that enable gut bacteria to produce therapeutic compounds, enhance nutrient absorption, or modulate immune responses can be achieved using CRISPR-Cas9 gene editing systems, transposon mutagenesis, and plasmid-based systems [
46]. One potential application of these modifications is the production of short-chain fatty acids, such as butyrate, which are known to have anti-inflammatory properties and play a critical role in maintaining gut health [
47]. The ability of engineered bacteria to increase the production of short-chain fatty acids could be used to alleviate the symptoms of inflammatory bowel disease and other inflammatory conditions. The genetically engineered bacteria could be engineered to produce enzymes that break down complex carbohydrates, improving nutrient absorption and potentially aiding in the management of metabolic disorders such as obesity and type 2 diabetes mellitus [
48].
The novel insights related to the ability of tardigrade-derived DSUP to induce radioprotection in transfected human cells [
49,
50,
51]. Building on this basic research, our idea was to take a significant step forward by transferring radioprotective proteins, mRNA and regulatory microRNAs directly into OMVs. This innovative approach aims to harness the natural protective mechanisms of tardigrades, known for their resilience in extreme environments, and apply them in a novel context. Using OMVs as delivery vehicles, we hope to create a more efficient and targeted method for delivery of these protective agents to human intestinal cells.