Maize is one of the world’s most important food and cash crops, as well as a key industrial raw material, cultivated extensively due to its considerable economic value. However, maize viral diseases pose a serious threat to global production [
1]. To date, over 40 distinct types of maize viruses have been reported worldwide [
2], significantly restricting the development of the maize industry [
3]. Of those, maize dwarf mosaic is particularly noteworthy, manifesting through symptoms such as dwarfing, chlorosis, and mosaic patterns on leaves. The primary causative agent of this disease is maize dwarf mosaic virus (MDMV), a single-stranded, positive-sense RNA virus belonging to the
Potyvirus genus. It is transmitted by aphids, rust spores, and seeds [
4]. Notably, the virus exhibits a relatively narrow host range, infecting only a small number of gramineous crops, such as maize and sorghum. MDMV is prevalent in maize-growing regions across Europe, America, Africa, and Australia. Recent studies suggested that its infection range is progressively expanding on a global scale [
1,
5,
6]. However, recent studies conducted by Chinese researchers indicated that the causative agent of maize dwarf mosaic disease in China is sugarcane mosaic virus (SCMV) rather than MDMV [
7]. China has classified MDMV as a quarantine pest under the List of Quarantine Pests of Imported Plants. During early infection, MDMV mainly impacts the leaves, leading to yellow streaks, chlorosis, and varying degrees of mosaic patterns. This is very similar to the symptoms of other major viruses that infect maize. As the disease progresses, affected plants exhibit stunted growth, reduced heading rates, lower pollen viability, and poor ear development [
2]. The virus not only infects maize independently but can also co-infect with maize chlorotic mottle virus (MCMV), wheat streak mosaic virus (WSMV), and SCMV, resulting in maize lethal necrosis (MLN). This condition poses a significant threat to production stability and the economic viability of maize-growing regions worldwide [
8].
Currently, detection of plant viruses is typically conducted using methods such as indicator plant identification, electron microscopy, serological assays, molecular biology techniques, and high-throughput sequencing [
9,
10,
11,
12,
13]. However, during the quarantine process, traditional methods like indicator plant identification and electron microscopy for detecting MDMV are both time-intensive and labor-intensive. While serological methods, such as ELISA, enable the simultaneous testing of a large number of samples, they are susceptible to cross-reactivity [
14]. Molecular techniques for MDMV detection, including RT-PCR, digital RT-PCR [
15], RT-LAMP [
16], and next-generation sequencing (NGS) technologies [
17], have been developed to address these challenges. Notably, NGS offers exceptional accuracy but remains economically unfeasible for routine diagnostic use due to its high cost [
18].
Mutations, genetic drift, and selective pressures can cause viruses, especially RNA viruses, to undergo a variety of sequence variations. As a member of the
Potyvirus genus, MDMV shares significant genetic similarity with other potyviruses in the same group [
19,
20], and as an RNA virus, it has high mutation rate [
2], which complicates accurate identification through single serological assays or conventional PCR methods. To address this challenge, a multi-gene combined detection technology has been developed, targeting multiple viral gene fragments [
21].
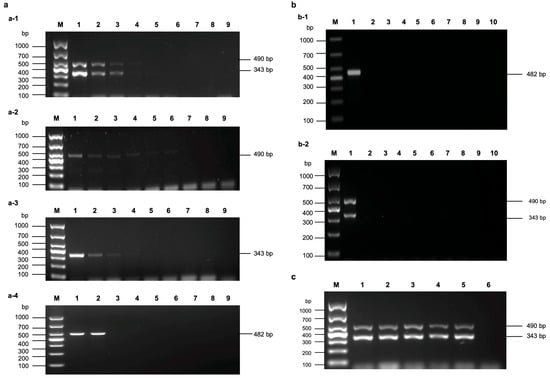
The multi-gene combined RT-PCR assay enables the simultaneous amplification of the conserved CP and CI genes of MDMV in a single reaction system. By targeting both genes concurrently, it overcomes the issue of false-negative results that are often caused by variations in individual gene fragments. This dual amplification strategy improved the accuracy and reliability of detection, facilitating more precise and rapid identification of MDMV. In this study, an RT-qPCR assay based on the CP gene was also developed to validate the practical application of the MDMV multi-gene combined RT-PCR assay. Collectively, these efforts provide essential technical support for maize quarantine measures and practical applications in agricultural production.
Source link
Yujie Jin www.mdpi.com