1. Introduction
Acute myocardial infarction (AMI) refers to myocardial tissue death resulting from an unstable ischemic condition [1]. The prognosis of AMI is closely related to its complications. Intracardiac thrombosis (ICT) is a rare but severe complication in AMI patients, involving pathological thrombus formation within the heart chambers, including the left atrial appendage [2,3]. Currently, no global reports exist on the incidence of ICT in AMI patients. A large-scale analysis of 11,724 autopsy reports found that 276 (2.4%) had ICT, with most deaths (73.3%) attributed to systemic embolism or pulmonary embolism [4]. Regarding intracardiac thrombi, research mainly focuses on the left ventricular thrombus (LVT) in AMI patients, with an incidence of about 4% [5,6]. The onset of ICT is often subtle, making early diagnosis challenging, yet it is associated with high mortality and serious complications. Detachment of the thrombus can lead to pulmonary embolism, cerebral infarction, and other events. Furthermore, the thrombus can impair cardiac function, resulting in heart failure or even cardiac rupture [7]. Therefore, early diagnosis of ICT in AMI patients is critical.
Advancements in diagnostic technologies have increased the detection rate of ICT among AMI patients [8]. However, most studies focus on symptomatic embolic events, which are typically diagnosed after other severe complications have occurred, potentially underestimating the true incidence of ICT. Transthoracic echocardiography (TTE) is the most used diagnostic tool, but its accuracy is limited by operator variability [9]. Delayed-enhancement cardiac magnetic resonance imaging (DE-CMR) is considered the gold standard in ICT diagnosis but its high cost limits its routine clinical use [10]. Given the limitations of current diagnostic methods, early screening and management of high-risk ICT patients remain complex and urgent issues.
Currently, most studies on this topic focus on exploring the risk factors of LVT after AMI [11]; there are no clinical studies that specifically examine the risk factors of ICT in AMI patients. Therefore, identifying specific risk factors of ICT in AMI patients and developing reliable predictive models are of paramount importance. Our study aims to analyze hospital examination results of AMI patients and develop a nomogram-based prediction model for ICT.
4. Discussion
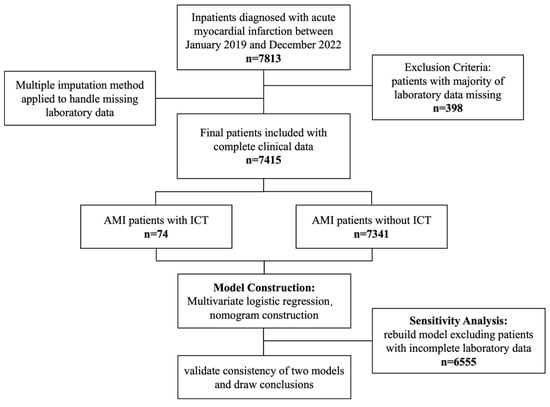
We included 7415 patients diagnosed with AMI during hospitalization, of whom 74 developed ICT. The independent risk factors of ICT after AMI were identified as male gender, anterior wall infarction, presence of ventricular aneurysm, and lower prothrombin activity. Based on these four risk factors, we developed a robust nomogram prediction model.
Gender plays a pivotal role in the development of ICT. Our study demonstrates that male patients are at significantly higher risk of ICT compared to female patients, which aligns with findings from prior research. A meta-analysis shows that male patients with AMI experience a higher incidence of thromboembolic complications, including ICT, with an adjusted odds ratio (OR) of 1.6. This underscores the gender-related disparity in ICT incidence following AMI [16]. Several underlying mechanisms may contribute to this increased risk. First, the protective effects of estrogen are a critical factor. Estrogen enhances the activity of anticoagulant proteins, such as protein C, improves endothelial function, and inhibits platelet aggregation [17]. The absence of estrogen in males removes this cardiovascular protection, thereby increasing the risk of thrombosis [18]. Furthermore, males may exhibit less favorable endothelial function, rendering them more susceptible to endothelial injury, a key initiator of thrombosis [19,20]. Anatomically, male hearts are typically larger and have thicker ventricular walls, which lead to higher blood flow velocity and increased shear stress on the vascular endothelium. This heightened shear stress contributes to endothelial damage, thereby raising the likelihood of ICT formation [21,22]. Additionally, lifestyle factors, such as higher rates of smoking, alcohol consumption, and poor dietary habits among men, exacerbate these risks [18]. In conclusion, male gender is a robust predictor of ICT risk. This finding emphasizes the need for gender-specific risk assessments in clinical practice and highlights the importance of tailored prevention and treatment strategies to address gender-based disparity in ICT outcomes.
AWMI is a significant risk factor of ICT, consistent with previous studies. In a prospective study by Andre Keren et al., 31% of patients with AWMI developed thrombus prior to discharge, compared to none in the inferior wall MI group (p < 0.001) [23,24]. Involvement of the left anterior descending artery (LAD) in AWMI leads to extensive myocardial injury and necrosis of the anterior wall, impairing left ventricular function. This myocardial necrosis induces local wall motion abnormalities, contributing to blood stagnation and elevating the risk of thrombus formation [25]. Post-infarction structural remodeling triggers hemodynamic changes, creating low-flow regions near the infarct zone, which are high-risk areas for thrombosis [25,26]. Additionally, AWMI can cause ventricular electrophysiological changes, including atrial fibrillation (AF), which is closely associated with thrombus formation due to irregular atrial contractions and blood stasis [27]. This relationship underscores the strong association between AWMI and elevated ICT incidence, highlighting the importance of targeted monitoring. Implementing routine thrombus screening and proactive anticoagulation therapy in patients with AWMI could further reduce ICT incidence.
Ventricular aneurysm significantly differed between the ICT and control groups in our study. Andre et al. identified ventricular aneurysm as an independent risk factor of thrombus formation during hospitalization, with 13 out of 130 patients developing new thrombi over a six-month follow-up period [23]. Ventricular aneurysm, primarily caused by AWMI, leads to myocardial injury, scar formation, and ventricular wall bulging. This results in increased myocardial compliance, impaired contractile function, and blood stasis. Previous studies have linked local contractile dysfunction in the ventricular aneurysm region to ICT formation [28]. Reduced blood flow and disturbed hemodynamics in this area are key mechanisms for thrombus development [25]. Additionally, ventricular aneurysm alters ventricular structure, promoting thrombus retention through fibrosis and scarring, which provide surfaces for thrombus adhesion and growth [29]. Long-term anticoagulation is often required in AMI patients with ventricular aneurysm to manage chronic blood stasis, necessitating more aggressive anticoagulation to prevent thrombus recurrence. Optimizing anticoagulation therapy by adjusting dosages and duration based on individual patient risk profiles could enhance thrombus recurrence prevention in these patients [30].
Our analysis revealed a significant association between reduced prothrombin activity and ICT occurrence, highlighting the role of coagulation dysfunction in ICT pathogenesis. Previous studies have shown a correlation between abnormal coagulation markers and myocardial injury. For instance, a study of 987 STEMI patients found a significant correlation between peak TnT and D-dimer and F1 + 2 as well as between NT-proBNP and D-dimer and F1 + 2 [31,32]. Myocardial injury contributes to thrombus formation through decreased coagulation capacity, which weakens stable clot formation and promotes unstable clots prone to fragmentation and reformation, increasing ICT risk [33]. Additionally, lower prothrombin activity may exacerbate thrombus formation by modulating the inflammatory response, as thrombosis in AMI patients is closely associated with inflammation markers like C-reactive protein (CRP) and interleukins, which enhance thrombotic activity [34]. Targeting prothrombin activity through therapeutic interventions, such as prothrombin complex concentrates or novel anticoagulants, may be strategies to stabilize clot formation and reduce ICT incidence in AMI patients.
Compared to previous clinical research on similar topics, our study presents several significant advantages. First, we developed a nomogram integrating four key risk factors—male gender, AWMI, ventricular aneurysm, and reduced prothrombin activity—which consolidates multiple risk factors into a single, user-friendly model. This enables precise prediction of ICT risk through simple calculations, facilitating clinical decision-making and personalized medicine. Second, our large sample size enhances the model’s applicability across diverse patient populations. Third, multiple validation methods, including ROC curve analysis and calibration curves, confirm the model’s accuracy and robustness. Sensitivity analyses further support the model’s reliability, highlighting its potential utility in improving clinical outcomes. The nomogram is a practical tool for clinicians to identify high-risk patients and tailor management strategies accordingly. For patients with a high predicted risk of ICT (e.g., a nomogram score ≥ 150), we recommend a comprehensive approach including the following: 1. Imaging surveillance: regular transthoracic echocardiography (TTE) to monitor thrombus formation, particularly in patients with anterior wall infarction or ventricular aneurysm. 2. Prophylactic anticoagulation: the initiation of low-molecular-weight heparin (e.g., enoxaparin) in high-risk patients without contraindications. 3. Therapeutic anticoagulation: an escalation to therapeutic anticoagulation (e.g., warfarin or direct oral anticoagulants) if a thrombus is detected, following current guidelines [3]. This integrated approach can help reduce the incidence of ICT and improve outcomes in high-risk AMI patients.
This study has several limitations. First, although our sample size of 7415 AMI patients is substantial, it is confined to a single center, which may limit the generalizability of the findings. Second, the ICT incidence rate of 1% is lower than that reported in other studies (5–8%) [35,36]. This may be due to the lack of recent prevalence data as well as advancements in treatments that reduce ICT incidence. Moreover, as a leading medical center in Northwestern China, our center’s timely interventions have significantly lowered complication rates. Additionally, the retrospective design introduces potential selection and information biases. Future larger-scale multi-center studies are needed to validate these findings and address regional variations. Despite these limitations, this study is the first to investigate ICT risk factors in AMI patients, filling a significant gap in research.
Source link
Xiaowei Huo www.mdpi.com