1. Introduction
In the modern world, there is tremendous growth in the agricultural field by incorporating new methods to increase the yield, to keep it fresh for a long time and to produce hybrid varieties. Hence, to cope with these tasks, pesticides in various forms (herbicide, insecticide, fungicide) have been used in a considerable amount. Carbendazim (CBZ), chemically named methyl-2-benzimidazole carbamate, is a common pesticide that has been widely used in agriculture during pre- and post-harvesting to prevent damage to fruits and cereal crops (such as wheat, rice and cotton) affected by fungal diseases such as spot, mildew, mold, rot and scorch [1,2,3,4,5]. CBZ found in fruit peels and cereals is a potential human carcinogen, and if consumed by humans can lead to liver disease and chromosome aberration. CBZ contains a benzimidazole ring that cannot be degraded easily and its extensive use causes the residues to remain in food, water and soil, causing serious issues to human health and the environment. In general, a maximum residue level (MRL) of 0.01 mg/kg is applicable to all pesticides [6]. And therefore, designing a simple, fast and sensitive detection method to accurately measure the CBZ residues is in high demand [6,7,8,9].
There are several traditional analytical methods used for the detection of CBZ, including high-performance liquid chromatography (HPLC), gas chromatography–mass spectrometry (GC-MS), ultraviolet–visible spectroscopy (UV-Vis) and fluorescence spectroscopy. Nonetheless, all these techniques are time consuming. And so, electrochemical sensing has received much attention recently due to its high sensitivity, rapid analysis, low cost, user friendliness and portability [10,11,12,13,14,15]. Carbon-based materials such as graphene, carbon black and carbon nanotubes (CNTs) and metals including metal nanoparticles, metal oxides and transition metal oxides (TMOs) are widely used as electrochemical sensing materials for the detection of trace level of pesticides in food including fruits, vegetables and crops [16,17,18,19,20,21,22].
Gao et al. [23] used nano-porous gold (NPG) as an electrochemical sensor for the detection of two different pesticides, methyl parathion (MP) and carbendazim (CBM). The prepared NPG/GC (glassy carbon) electrode exhibits high sensitivity for MP (186.53 µA mM−1 cm−2) and CBM (484.51 µA mM−1 cm−2), and low detection limits (0.02 mM for MP and 0.24 mM for CBM). Additionally, it also provides high specificity, selectivity and anti-interference capabilities. Transition metal oxides and metal nanoparticles exhibit good electrical and photocatalytic properties, as they possess different shapes, structures, stability and large surface area. But their major drawback is their wide band gap, which reduces their conductivity and finally leads to pulverization of the electrode film. Hence, to overcome this issue, the metal particles are used along with a carbon matrix.
Ji et al. [21] prepared a novel electrochemical sensor based on multiwalled carbon nanotubes (MWNTs) and Au nanoparticles (AuNPs) on molecularly imprinted polymer (MIP) membranes for the clinical diagnosis of cholesterol. The MIP sensor showed a linear response range between 10−13 and 10−19 mol L−1 and the limit of detection (LOD) was found to be 3.3 × 10−14 mol L−1. Anshori et al. [18] synthesized a bio-sensing material based on functionalized multi-walled carbon nanotube/silver nanoparticles (f-MWCNT/AgNPs) for the detection of dopamine exhibiting an LOD value of 0.2778 mM in the linear range of 0–8 mM. Wang et al. [20] proposed a low-cost ultrasonic assisted strategy and prepared graphitized and carboxylated carbon nanotubes along with cerium oxide, GR-MWCNT-COOH@CeO2/GCE sensor for the detection of methyl parathion with a lower LOD value of 0.0285 mM. Wu et al. [22] prepared palladium-multiwalled carbon nanotube composites (Pd-MWCNTs) as a sensing platform for acetaminophen showing an acceptable linear response between 0.5 and 100 µM with a detection limit of 0.13 µM. Gao et al. [17] synthesized gold and zirconia nanocomposite-modified graphene nanosheets (Au-ZrO2-GNs/GCE) for the electrochemical detection of methyl parathion showing a very low LOD of 1 ng mL−1.
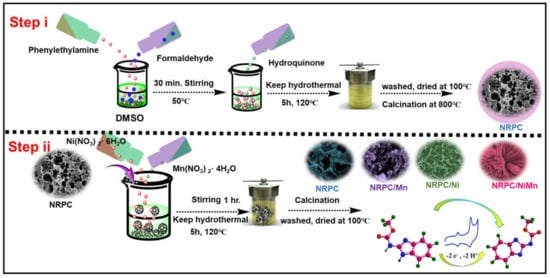
Based on the above-mentioned reports, we proposed the combination of carbon material and transition metal oxide for the detection of carbendazim. The carbon material, namely, nitrogen-rich porous carbon (NRPC), was synthesized from polybenzoxazine and the transition metal inclusion into NRPC, i.e., NRPC/Ni, NRPC/Mn and NRPC/NiMn, was carried out with subsequent methods involving carbonization, activation and hydrothermal reactions. We believe that the synergistic effect produced from the combination of NRPC and transition metal(s) could improve the electrochemical sensing performance for the detection of CBZ.
3. Results and Discussions
The structure and morphology of the synthesized materials (Figure 1), NRPC, NRPC/Mn, NRPC/Ni and NRPC/NiMn, were characterized and analyzed with different methods including Raman, XRD, BET, XPS, SEM and TEM analyses. The Raman spectra of the prepared materials are given in Figure S1a. The NRPC material shows two bands at 1354 and 1586 cm−1, respectively, denoting the ‘D band’ and ‘G band’. The ‘D band’ is due to the degree of disorderedness and the ‘G band’ is due to the degree of graphitization. The other materials, NRPC/Mn, NRPC/Ni and NRPC/NiMn, show a third band at 534 cm−1, due to M-O-M bonds, in addition to the D and G bands. The ratio of the intensity of the ‘D band’ (ID) to the intensity of the ‘G band’ (IG) gives an idea of the extent of disorderedness produced. It could be observed that the ID/IG value is larger for NRPC/NiMn, indicating increased disorderedness [23,24].
The XRD patterns of the prepared materials are given in Figure S1b. The pristine carbon material, NRPC, shows two diffraction peaks at 24.2 and 44.6°, respectively, corresponding to the (002) and (001) planes of the carbon materials. NRPC/Mn, NRPC/Ni and NRPC/NiMn show additional diffraction patterns corresponding to the (003), (101), (012), (015) and (018) planes of the rhombohedral phase. The property of the prepared materials was analyzed with BET analysis. Figure S1c,d display the N2 adsorption/desorption isotherm and pore size distribution (PSD) of the given materials. The N2 adsorption/desorption isotherms show type IV isotherm with an H3 hysteresis loop denoting mesoporous materials. The PSD curves indicate the presence of both micro- and mesoporous materials with pore diameter ranging between 2 and 50 nm [25,26].
X-ray photoelectron spectroscopy (XPS), a powerful analytical technique, was employed to unravel the elemental makeup and intricate chemical interactions occurring on the surface of NRPC/NiMn materials. This investigation yielded invaluable data, shedding light on the material’s fundamental characteristics and paving way for future advancements. Figure 2a–f showcase high-resolution XPS spectra, akin to chemical fingerprints, for C 1s, N 1s, O 1s, Mn 2p and Ni 2p. These spectra meticulously detail the symphony of elements present within NRPC/NiMn, offering a comprehensive perspective on its building blocks. Notably, the absence of any extraneous peaks underscores the exceptional purity of both NRPC and NRPC composite materials. To delve deeper into the interplay between various atoms, the XPS survey scan was deconvoluted and is shown in Figure 2b–f. This scan unveils distinct peaks corresponding to each element—carbon (C), nitrogen (N), oxygen (O), manganese (Mn) and nickel (Ni)—at characteristic binding energies of approximately 285, 399, 533, 643 and 860 eV, respectively. These unique energy signatures act as identifiers for each element, akin to a chemist’s fingerprint library. Zooming in on the C 1s spectrum (Figure 2b) reveals a captivating story. Four distinct peaks emerge, each representing a specific chemical environment surrounding the carbon atoms. These peaks meticulously map various carbon-based bonds, including hydrocarbon chains (C–C and C=C) at 286.5 and 285.2 eV, carbon–nitrogen bonds (C–N) at 288.2 eV and carbons linked to oxygen-containing groups (C=O) at 285.6 eV. By deciphering these peaks, scientists can unveil the intricate molecular structure and composition of the material. Similarly, the XPS technique sheds light on the diverse configurations of nitrogen atoms within NRPC/NiMn. The N 1s spectrum unveils a fascinating array of peaks at varying binding energies, each corresponding to a specific type of nitrogen species. Graphitic and pyrrolic N can be identified by their unique energy signatures at 399.4 and 400.5 eV, signifying the presence of a rich tapestry of nitrogen functionalities within the material. Interestingly, the nitrogen species originating from the polybenzoxazine precursor are known to exhibit enhanced electrochemical activity, contributing to a significant boost in capacitance, particularly in acidic environments. XPS analysis also unveils the intriguing interplay between oxygen and carbon atoms. The O 1s spectrum resolves the oxygen bonding into distinct peaks at 532.9, 533.5 and 532.5 eV, each representing quinone and phenolic hydroxyl groups. These functional groups play a critical role in defining the material’s properties. Notably, phenolic hydroxyl groups are particularly noteworthy for their ability to facilitate high pseudo-capacitance through reduction reactions, ultimately enhancing the material’s overall performance [27,28]. Delving deeper, the Mn 2p spectrum unveils a captivating story of manganese. The Mn 2p spectrum (Figure 2e) reveals four peaks showing different states of Mn. The peaks at 641.5 and 652.5 eV correspond to Mn2+ 2p3/2 and Mn2+ 2p1/2, and the peaks at 642.5 and 653.5 eV correspond to Mn3+ 2p3/2 and Mn3+ 2p1/2. This analysis is crucial for understanding the electrochemical behavior of manganese in the material. Similarly, the Ni 2p spectrum (Figure 2f) shows peaks corresponding to Ni2+ 2p3/2 and Ni2+ 2p1/2 at 855.0 and 872.5 eV, respectively, along with satellite peaks at 861.5 and 880.0 eV, signifying different nickel species [29]. These peaks provide insight into the oxidation states of nickel within the material, which are important for its catalytic and electrochemical properties.
Scanning electron microscopy (SEM) was employed to gain a deeper understanding of how the microscopic structures of different materials evolve. These materials included porous carbon (NRPC), and composites where NRPC was combined with manganese (Mn), nickel (Ni) or both Mn and Ni (NRPC/NiMn). The resulting magnified images are presented in Figure 3. At its core, NRPC resembled a maze (labyrinthine network) riddled with numerous holes (pores) and empty spaces (voids). This intricate framework included few large pores (macropores) of different sizes scattered amongst the voids. Interestingly, the overall structure appeared somewhat compressed, hinting at a potential collapse. When manganese (Mn) was incorporated into NRPC (NRPC/Mn), a remarkable transformation occurred. The initially observed maze-like structure gave way to unique flake-like shapes with wider ends. In contrast, adding nickel (Ni) to NRPC (NRPC/Ni) produced a distinct “crushed petal” morphology with narrow, pointed ends (spike-like). It is worth noting that these petal formations seemed to be intricately woven into the existing porous carbon matrix of NRPC. The combination of both Mn and Ni (NRPC/NiMn) yielded particularly fascinating results.
The presence of these two elements triggered the formation of numerous interconnected petal-like structures. Remarkably, these petals exhibited a vertical growth pattern, eventually merging to create a hierarchical 3D flower-like architecture. This growth pattern implied a gradual building process, where an increasing number of petals densely packed together to form a cohesive structure. At the centre of this 3D flower-like structure resided a core containing empty spaces (voids). This core acted as a central hub for the petals, providing crucial support and maintaining the structural integrity of the entire 3D framework. This inner core played a critical role in ensuring the stability of the flower-shaped structure. Additionally, the interconnected voids within the core offered a multitude of electroactive sites, which are essential for storing ions in supercapacitor electrodes [23,24].
To investigate the morphology and elemental composition of the bimetallic species embedded within the porous carbon matrix (NRPC/NiMn), we employed Transmission Electron Microscopy (TEM) techniques. The TEM image offers a high-resolution view on a nanoscale (Figure 4a–c), clearly revealing the porous carbon structure hosting well-dispersed clusters of metallic nanoparticles. This visualization provides direct evidence for the successful integration of the desired bimetallic component within the carbon matrix. Further confirmation of the material’s composition came from SAED and Energy-Dispersive X-ray Spectroscopy (EDX) mapping (Figure 4d–k). The diffraction rings corresponding to different planes, (220), (311) and (222), are evident from the SAED pattern. This technique identified a broad spectrum of elements, including carbon (C), oxygen (O), nitrogen (N), nickel (Ni) and manganese (Mn). This comprehensive elemental analysis aligns perfectly with our initial hypothesis. The presence of oxygen and nitrogen signifies their successful incorporation into the porous carbon scaffold, likely facilitated by the use of Pbz as the carbon source. Moreover, the detection of both nickel and manganese elements confirms their intended integration into the carbon framework. This successful incorporation of all targeted elements from the Pbz precursor and bimetals represents a crucial step forward in tailoring the material’s property for enhanced electrochemical performance. This innovative approach, combining Pbz-derived carbon with embedded bimetallic species, establishes a valuable benchmark for future research efforts. It paves the way for further exploration aimed at optimizing the performance and efficiency of energy-storage devices like supercapacitors. This advancement has the potential to significantly impact various technological applications that rely on high-performance energy-storage solutions.
Source link
Shakila Parveen Asrafali www.mdpi.com