1. Introduction
Aeromonas hydrophila is a ubiquitous pathogen within aquatic ecosystems and has led to substantial economic burdens for aquaculture [
1,
2,
3]. Several prominent virulence factors have been identified, such as Type Three Secretion Systems (T3SSs) and T6SSs; toxins like Alt (heat-labile cytotonic enterotoxin), Act (cytotoxic enterotoxin), Ahp (serine protease); and various hemolysins [
4]. In addition, siderophores are crucial for
A.
hydrophila to override the iron limitations imposed by the host or the environment [
5]. Amonabactins, siderophores produced by the
Aeromonas genus, can be used for iron acquisition to support a bacterium’s growth [
6,
7]. A previous study showed that
A.
hydrophila without the amonabactin biosynthesis gene
AmoG was unable to synthesize any amonabactin or grow under iron stress conditions [
8]. Nonetheless, the direct impact of
A. hydrophila-produced amonabactin on host infection dynamics and its potential roles in modulating the fish epithelial barrier remain unexplored.
The integrity of the gut epithelial barrier is critical for preserving gut functionality and systemic immunity [
9]. Pathogens employ different virulence mechanisms to disrupt epithelial junctions, thereby undermining gut barrier integrity [
10,
11,
12].
A. hydrophila, notorious for its ability to severely compromise gut integrity and trigger septicemia, has been observed to cause gut lesions and induce gut inflammation in fish [
13,
14,
15]. In a previous study, we identified a secretory serine protease (Ssp1) from
A. hydrophila that plays a critical role in disrupting the gut’s tight junction barrier [
16]. In addition, our earlier work pinpointed WbuB, a GT4 galactosaminogalactan synthase from
A. hydrophila, as vital for inflammasome activation and gut tight junction disruption [
17]. Nevertheless, the specifics of how
A. hydrophila exploits nutrient acquisition, notably through amonabactin, to impact the gut mucosa barriers remain unclear.
In this study, we identified an amonabactin cluster with a potential iron uptake ability in A. hydrophila CCL1 (GenBank Accession No. CP092356). To elucidate this pathogenic mechanism, we selected the amonabactin biosynthesis gene AmoG for further study to determine whether it is a virulence factor in vivo. Through engineering ΔAmoG and its complement strain, comparative analyses encompassing physiological attributes and virulence capacities were conducted for these strains. Moreover, in vivo studies suggested that A. hydrophila may use amonabactin as one of its virulence factors to inhibit the Wnt/β-catenin pathway, consequently impairing the structure and function of the gut mucosal barrier. Our findings suggested that, unlike Ssp1 and WbuB, which predominantly influence the tight junction and inflammasome pathway, AmoG seems to manipulate the host Wnt/β-catenin signaling pathway through nutrient acquisition. These results provide key insights into gut mucosal dysfunction during A. hydrophila infection.
2. Materials and Methods
2.1. Fish
Crucian carps (~14.2 g) were obtained from Hunan Yuelu Mountain Science and Technology Co., Ltd.: Changsha, China, which carries out aquatic breeding in Changsha. During the experiment, fish were housed pre-experimentally under controlled conditions (25.25 ± 0.58 °C, pH 7.05 ± 0.12, >7.0 mg/L O
2). Pre-experimental screening confirmed that the fish were disease-free [
18]. Tissues were acquired following ethical guidelines (GB/T 35892-2018 [
19]). All animal experiments were approved by the Hunan Normal University’s Animal Care Committee (No. 2023109).
2.2. Generation of A. hydrophila ΔAmoG and ΔAmoG-C
To generate the
A. hydrophila Δ
AmoG mutant, a 300 bp in-frame deletion of AmoG (aa 661–760) was engineered via an overlap extension PCR. The primers AmoG-F1/AmoG-R1 and AmoG-F2/AmoG-R2 were used sequentially for overlap PCRs, followed by a fusion PCR using AmoG-F1/AmoG-R2 (
Table S1). The resultant amplicon was ligated into pDM4 at the BglII site, yielding pDMAmoG.
Escherichia coli S17-1 λpir harboring pDMAmoG was conjugated with
A. hydrophila CCL1. Following coculture and selection on polymyxin B/chloramphenicol plates, sucrose-resistant yet chloramphenicol-sensitive colonies were isolated. Confirmation of in-frame deletion in these isolates was achieved through PCR and sequencing, leading to the identification of the Δ
AmoG mutant.
For the creation of the AmoG complement strain, designated Δ
AmoG-C, the gene encoding AmoG, along with its native promoter, was PCR-amplified using the primers AmoG-F3 and AmoG-R3 (
Table S1). This amplicon was then cloned into the pACYC184 plasmid at its EcoRV restriction site, forming pACYCAmoG-C. Subsequently, this construct was introduced into
E. coli S17-1 λpir, which was subsequently mated with the Δ
AmoG mutant. Transconjugants were identified on selective LB agar plates containing polymyxin B and chloramphenicol. One selected colony, designated Δ
AmoG-C, was further validated by PCR and sequence confirmation.
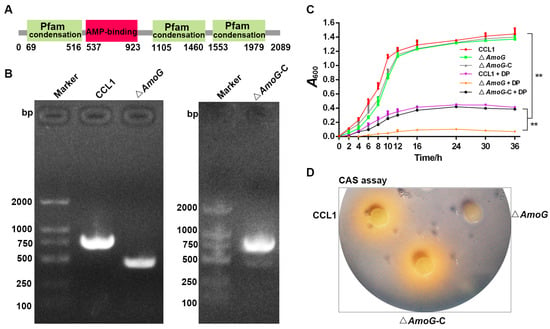
2.3. Characterization of ΔAmoG and ΔAmoG-C
The strains CCL1, Δ
AmoG, and Δ
AmoG-C were cultured in LB broth at 28 °C for 24 h. Scanning electron microscopy was employed to assess cell morphology. Growth dynamics were monitored by cultivating the strains in LB with or without 100 μM 2, 2′-dipyridyl (Dp), starting at an A600 of 0.01; samples were taken at various intervals to measure A600. Motility assays and biofilm formation tests were conducted according to Reference [
16]. The Chrome Azurol S (CAS) plate assay and Arnow’s test were used to analyze siderophore production according to Reference [
20]. Each assay was replicated three times.
2.4. Cellular Adherence and Cytotoxicity Analyses
Caco-2 cells, obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), were maintained in DMEM enriched with 20% FBS and antibiotics (penicillin/streptomycin) at 37 °C in 5% CO2. Approximately 1 × 105 Caco-2 cells were seeded into 96-well plates and infected with CCL1, ΔAmoG, ΔAmoG-C, or PBS (control) at an MOI of 10:1. Cells were incubated for 1 h before washing away unbound bacteria using PBS. After infection, cells were lysed with Triton X-100, and the lysates were spread on LB agar to determine the bacterial counts. The adhesion index was calculated as the ratio of associated bacteria to Caco-2 cells. Each assay was replicated three times. Cytotoxicity was measured by the lactate dehydrogenase (LDH) method. Briefly, after 1 h of infection as above, LDH leakage was assessed using a Cytotoxicity Detection Kit (Roche Diagnostics, Mannheim, Germany). Cells treated with Triton X-100 represented the maximum LDH release (100%). Each assay was replicated three times.
2.5. In Vivo Infection
For in vivo infection, fish were injected intramuscularly (i.m.) with 100 µL suspensions containing 1 × 10
6 CFU/mL of CCL1, Δ
AmoG, Δ
AmoG-C, or an equivalent volume of PBS (control). At 24 h post infection (hpi), distal gut, kidney, spleen, and blood from CCL1-, Δ
AmoG-, Δ
AmoG-C-, or PBS (control)-treated fish were carefully sampled under sterile conditions for subsequent experimental procedures. (i) Bacterial dissemination in the kidney and spleen and AB-PAS staining were carried out as reported previously [
21]. (ii) A qRT-PCR was performed to analyze the genes’ expression in the distal gut. The genes and PCR primers used are listed in
Table S1. Expression levels were measured through the 2
−ΔΔCT method, referencing beta-actin, with PCR efficiency (E) and correlation (R
2) checked as previously detailed [
22]. (iii) Plasma was derived from the blood of each group. D-lactic acid concentrations were measured using a D-lactic acid ELISA assay kit procured from Nanjing Jiancheng Bioengineering Institute, Nanjing, China. Additionally, Limulus Amebocyte Lysate (LAL) QCL-1000 kits from Lonza were employed to quantify lipopolysaccharide (LPS) levels within the plasma. (iv) An immunohistochemistry (IHC) assay was used to analyze the expression of Occludin in the distal gut as reported previously [
16]. Mouse anti-rOccludin antibody (prepared in [
16]) and Cy3-conjugated goat anti-mouse secondary antibody (Servicebio, Wuhan, China) were used as the primary and secondary antibodies, respectively. (v) Iron concentrations in the serum and distal gut were determined using an iron-unsaturated iron binding capacity kit (Nanjing Jiancheng) according to the manufacturer’s instruction. The DAB-enhanced Prussian blue iron staining kit (Servicebio, Wuhan, China) was utilized to detect iron deposits within liver samples according to the manufacturer’s instructions. (vi) To conduct an apoptosis assessment targeting the distal guts, an apoptosis assay was carried out using the TUNEL Apoptosis Detection Kit (Servicebio). (vii) For assessing survival rates, twenty crucian carps per group were challenged with CCL1, Δ
AmoG, Δ
AmoG-C, or received a PBS control, following the same inoculation procedure as described above. Over a period of two weeks, fish mortality was meticulously documented post infection as reported previously [
21]. Each assay was replicated three times, with three fish used each time.
2.6. Gut Transcriptome and Microbiome Analyses
Transcriptome analysis was performed as outlined in previous work [
16]. At 24 hpi, as above, distal guts from fish infected with CCL1, Δ
AmoG, Δ
AmoG-C, or exposed to PBS (serving as control) were collected under sterile conditions (with four fish in each group). Gut specimens from each group were frozen at −80 °C prior to RNA extraction for subsequent RNA sequencing (RNA-Seq). RNA extraction, library construction, and data analysis were performed by Novogene (Beijing, China).
For a comprehensive analysis of the microbiome shifts in the guts of fish exposed to CCL1, Δ
AmoG, Δ
AmoG-C, or treated with PBS (control), as above, distal guts from each group were taken and forwarded to Novogene for expert processing and sequencing (with four fish in each group). Following CTAB/SDS DNA extraction, libraries were constructed and sequenced using the 515F and 806R primer pair on an Illumina NovaSeq6000. Rigorous data analysis was performed through QIIME2 as reported previously [
16].
2.7. Effect of the Activation of the Wnt/β-Catenin Pathway on Infection
Fish (twenty fish in each group) were injected i.m. with 100 µL of the Wnt/β-catenin agonist BIO (0.5 mg/mL, Sigma, St. Louis, MO, USA). Two hours following the initial procedure, each fish received an intramuscular injection comprising 100 µL of suspension, with concentrations of 1 × 106 CFU/mL for either CCL1, ΔAmoG, ΔAmoG-C, or PBS. At 24 hpi, the kidney, spleen, and blood from CCL1-, ΔAmoG-, ΔAmoG-C-, or PBS (control)-injected fish were carefully sampled as above. Plasma LPS levels, bacterial dissemination, and survival rate assays were determined as reported above. Each assay was replicated three times.
2.8. Statistical Analysis
The sample size was calculated using PASS software (PASS 11. NCSS, LLC. Kaysville, UT, USA). Data were statistically analyzed using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) and the Unpaired Mann–Whitney test or the Log-Rank test.
4. Discussion
Iron is an essential trace element for most organisms [
25,
26,
27]. In response to iron starvation,
A.
hydrophila has been shown to possess multiple systems for the sequestration of host iron, including heme-bound iron transport [
28], the utilization of enterobactin siderophores produced by Enterobacteriaceae [
6,
7], and the secretion of amonabactin [
29]. Among these virulence factors, amonabactin represents a family of catechol peptidic siderophores that is composed of seven genes named
AmoCEBFAGH [
8]. A previous study indicated that a mutant of AmoG was unable to synthesize any amonabactin or to grow in iron-stress conditions [
8]. Consistent with this study, our results showed that under a treatment with 100 µM Dp, Δ
AmoG was almost unable to grow, indicating that the AmoG of
A.
hydrophila CCL1 may be involved in bacterial iron acquisition and survival under iron stress.
Until now, AmoG’s in vivo function has remained unexplored experimentally. To explore this, firstly, we embarked on a comprehensive transcriptomic analysis targeting the guts of crucian carps infected with
A. hydrophila. Our findings unveiled that in comparison to Δ
AmoG infections, CCL1 and Δ
AmoG-C infections markedly suppressed the expression of pivotal genes in the Wnt/β-catenin signaling pathway including
wnt10b,
axin2,
ccnd1, and
ctnnb1. The Wnt/β-catenin pathway is integral to the structural integrity of the gut barrier and is essential for the proper functioning of gut epithelial cells, such as goblet cells [
30]. In line with the above observations, we observed a significant decline in goblet cells and
MUC2,
IL-22,
Hepcidin-1, and
LEAP-2 expression in fish subjected to CCL1 or Δ
AmoG-C infection. These results indicated that AmoG may be involved in the pathogenicity of
A.
hydrophila infection through the inhibition of the Wnt/β-catenin pathway. Secondly, the Wnt/β-catenin pathway promotes the assembly and stability of gut tight junctions, ensuring minimal paracellular leakage [
31,
32]. Previous investigations have documented the capacity of specific pathogens, notably
Helicobacter pylori and
Campylobacter jejuni, to inhibit the Wnt/β-catenin pathway and compromise the integrity of tight junctions during the course of their infection [
33]. For this purpose, we analyzed the impact of AmoG on gut permeability in an in vivo setting. Our findings revealed a significant upregulation of occludin in fish infected with Δ
AmoG. Consistently, the decreased concentrations of plasma D-lactic acid and LPS indicated a reduced gut mucosal permeability following Δ
AmoG infection. Moreover, our data demonstrated a slight but notable enhancement in alpha diversity within the gut flora of fish infected with Δ
AmoG relative to those harboring CCL1, hinting at potential disturbances in bacterial colonization elicited by AmoG [
34,
35]. Collectively, the above results suggested that AmoG might be involved in the infection process, particularly under conditions of an inhibited Wnt/β-catenin pathway and disrupted tight junction barriers. However, additional studies are warranted to validate whether AmoG’s effects are direct or mediated indirectly through other pathways.
Iron is an indispensable micronutrient for bacterial metabolism, proliferation, and infection. Bacterial pathogens have developed intricate systems for iron acquisition, employing iron carriers known as siderophores to chelate and sequester iron from the host environment, influencing their infection capacities [
36,
37]. Similarly, our results demonstrated that Δ
AmoG infection leads to elevated iron levels in the serum, gut, and liver of infected fish, possibly due to its impaired iron-chelating efficiency, which may subsequently affect the infection dynamics of
A. hydrophila. Indeed, upon infecting fish with Δ
AmoG, we observed notably smaller bacterial counts in their tissues compared to those in fish infected with
CCL1. The reason may be that the iron-chelating process used by
A. hydrophila enables the bacterium to disrupt the gut barrier and simultaneously enhance its invasive capabilities. Research suggests that the inhibition of the Wnt/β-catenin signaling pathway by pathogens through iron chelation provides the bacteria with an advantage in disrupting the gut barrier, thereby enhancing their invasiveness [
38]. Studies have reported that a shortage of iron, brought about by siderophores, can lead to the suppression of the Wnt/β-catenin signaling pathway, favoring bacterial infection [
39,
40]. Consistent with these findings, our findings revealed that once the Wnt/β-catenin pathway is activated, the presence or absence of amonabactin does not seem to significantly alter the bacterium’s infectivity. This observation further points to the possibility that
A. hydrophila may use amonabactin as one of its virulence factors to inhibit the Wnt/β-catenin pathway and consequently impair the structure and function of the gut mucosal barrier. In addition, a study on
Edwardsiella piscicida highlights the role of Fur in iron regulation and virulence, similar to the role of AmoG in
A. hydrophila. In the future, using siderophore-deficient strains as vaccines may be an effective prevention and control strategy in aquaculture [
41].
Interestingly, our previous studies have identified Ssp1 and WbuB as essential components for disrupting the gut’s tight junction barrier [
16,
17]. In contrast to Ssp1 and WbuB, which primarily influence the tight junction and inflammasome pathway, AmoG uniquely targets the Wnt/β-catenin signaling pathway. Through iron acquisition, AmoG appears to manipulate the host’s Wnt/β-catenin signaling pathway, thereby facilitating both pathogenesis and nutrient acquisition. Further exploration is needed to understand the precise mechanisms linking iron chelation to the mediation of the Wnt/β-catenin signaling pathway.