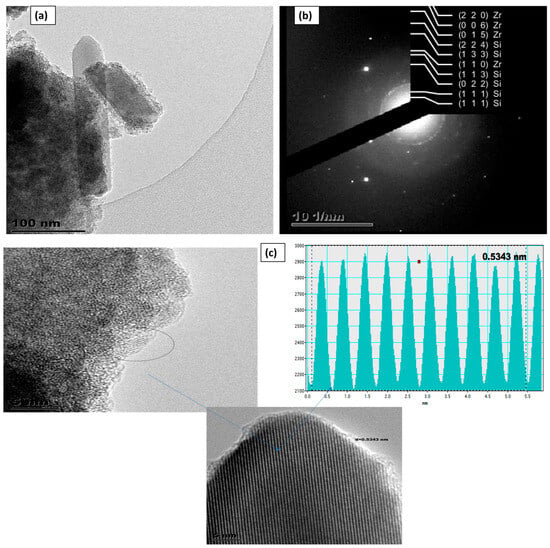
Nanotechnology has become an important field of study due to the unique chemical, biological, electrical, optical, and magnetic properties of nanomaterials [1]. Because the field of nanotechnology has so many uses, such as dental care, biological sensors, medical imaging, assays, diagnostic kits, athletic gear, sunscreens, cosmetics, environmental cleanup, textiles, and gene inactivation, many researchers focus on this field of study [1]. The growth of nanotechnology has enabled researchers to look for new materials and modify already-existing materials to create new procedures. One such approach is the levitation technique, which is a cheap, efficient, reasonably easy, and adaptable method of making materials [2]. Materials of sizes between 1 and 100 nanometres are known as nanoparticles and have special characteristics that distinguish them from bulk materials [3,4]. Their improved surface-to-volume ratio, quantum effects, and altered chemical and physical properties on a nanoscale are the sources of these properties [5,6]. Oxide compounds are not conductive to electricity; however, certain perovskite-structured oxides are electronically conductive, finding application in the cathodes of solid oxide fuel cells and oxygen generation systems. They are insoluble in aqueous solutions and extremely stable, making them useful in ceramic structures and as lightweight structural components in aerospace and electrochemical applications such as fuel cells in which they exhibit ionic conductivity. Zirconia oxide (ZrO2) finds extensive applications in a variety of fields, including biological fields (e.g., biosensors, cancer treatment, and hip replacement), optical coatings, solar cells, fuel cells, dentistry, oxidation selectivity, and catalysis [5,7,8,9,10]. Zirconia is an appealing material for numerous applications due to its exceptional qualities, including its redox, acid–basic, and chemical stability, as well as its mechanical resistance [5,7], high surface-to-volume ratio, and small size [8,9,10]. With a high melting temperature of 2750 °C, which makes it valuable as a refractory material, ZrO2 has a special combination of features that set it apart from all other ceramic materials [11]. Because of the low thermal conductivity of zirconia, it is a suitable choice for thermal protection coatings and insulation materials at high temperatures [12]. Zirconia is characterised by a high hardness and strength and is a durable material for applications that require mechanical stress resistance. ZrO2 can also be used as an additive to enhance the performance of materials, drug delivery, and medical imaging due to its biocompatibility [13]. This property makes it useful in applications related to oxygen sensing and in high-temperature fuel cells [14].
Zirconia is a special high-temperature solid electrolyte because it forms structural defects with oxygen ion vacancies upon doping with specific aliovalent oxides. This significantly increases oxygen ion conductivity [11]. Moreover, zirconia can provide good ionic conductivity and thermochemical stability when doped with different transition metal cations [15] and has shown the possibility of electrochemical measurement using solid-state galvanic cells based on zirconium solid electrolytes [16]. However, ZrO2 has limited applications in industry due to its low acidity, basicity, and porosity, as well as its low BET surface area of 50 m2/g compared to other oxide materials such as SiO2, Al2O3, and TiO2 [17]. Mesoporous silica has a high surface area and well-defined pore structure, ensuring sufficient active sites for catalytic reactions, adsorption processes, and drug delivery applications [18]. The combination of mesoporous silicon and zirconium oxide nanoparticles can achieve an optimal balance between surface area, conductivity, stability, pore size distribution, and mechanical stability, making it very useful for various applications such as electrical equipment, sensors, catalyst supports, and solar cells [7]. The zirconium oxide phase is shown to be more stable when combined with silicon dioxide (SiO2) due to the creation of Si-O-Zr bonds [19]. Furthermore, the benefits of ZrO2/SiO2 binary oxide include the synthesis of Si-OH (silanol) and Zr-OH groups, which operate as sites for increasing the formation tendency of an apatite layer. Silanol groups can connect to biomolecules, resulting in a multifunctional coating for drug delivery, cell targeting, bioimaging, and biosensing [19].
Source link
Rudzani Sigwadi www.mdpi.com