1. Introduction
Hearing loss (HL) is one of the most common sensory impairments worldwide and represents a critical medical and public health issue [
1]. Etiologies vary according to the age at onset of HL and include genetic, infective, toxic and environmental factors. Overall, genetic factors account for at least 40% of the cases, and a relevant portion of affected patients can have a definite molecular diagnosis thanks to Next Generation Sequencing (NGS) technologies [
2]. Inner ear malformations (IEMs) are known to be detectable in a proportion ranging from 30 up to 50% of children with congenital sensorineural hearing loss [
3,
4]. Furthermore, although newborns with hearing deficits can use auditory temporal information during the development in the first months of life [
5], it takes them longer to mature and process this information efficiently along the entire auditory pathway. If hearing loss is confirmed during clinical tests after the screening, hearing aids and eventually cochlear implants are effective in auditory rehabilitation; this is also true in the case of inner ear malformations [
6].
Neonatal hearing screening is an early hearing-detection and intervention strategy which aims to identify infants with potential conductive and sensorineural hearing deficits. The data in the literature strongly suggest that the early detection and rehabilitation of a hearing impairment are essential factors for the development of language and relative social and cognitive skills [
7,
8].
At present, two clinical methodologies are available to conduct neonatal hearing screening; these protocols are based on otoacoustic emissions (OAEs) or on automated auditory brainstem responses (AABRs) [
9,
10]. In terms of screening, the OAEs access the functionality of the inner ear by examining the functional status of the outer hair cells; thus, they provide an assessment of the auditory periphery. The AABR provides an indication of the auditory brainstem functionality; thus, the hearing assessment is peripheral and retro-cochlear.
OAEs represent a fast, non-invasive and cost-effective method; a small probe is placed in the neonatal ear canal, the microphones of the probe emit specific transient stimuli (i.e., clicks, chirps, tone busts) and after a short time, in ms, the same microphones record the acoustical echoes produced by a reflection of the stimulus energy within the inner ear. These echoes are generated by the nonlinear behavior of the outer hair cells on the organ of Corti [
9,
11]. Since these responses are low-level acoustical signals, there is a need to record them in silent clinical setups with very low ambient noise.
The AABR is an electrical response; it is generally more accurate than the OAEs in detecting hearing deficits and does not require a quiet environment, although some electromagnetic shielding is always recommended in order to avoid signal artifacts and long acquisition times [
10]. The AABR is recorded by three electrodes placed on the infant’s head, and specific algorithms detect the presence of wave V in the acquired response, providing a positive outcome of the test (PASS).
Overall, the cost of the AABR devices and the relative consumables are more expensive compared to OAEs. Usually, OAE screeners are used in the first phase of screening and subsequent evaluation by AABR follows in cases of technical OAE problems or REFER OAE results. This protocol combination has become the standard in audiological clinical practice [
12,
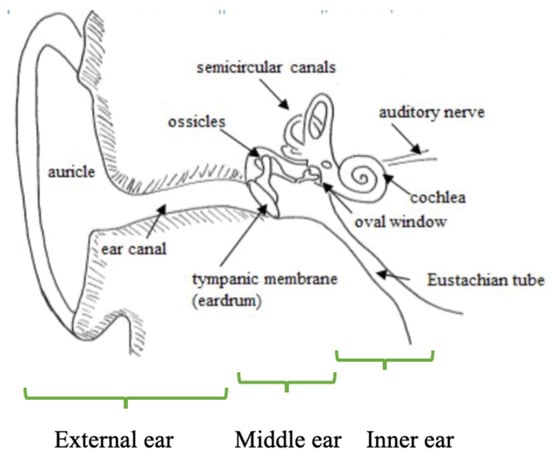
13]. The auditory periphery is depicted in
Figure 1.
The technologies involved in neonatal hearing screening have not evolved particularly in the last 30 years. In terms of OAEs, two different protocols are still being used, as in the 90s. These refer to TEOAEs evoked by a series of 80 clicks and DPOAEs evoked by asymmetrical pure tone stimuli, such as 65- and 55-dB SPL, having a frequency ratio of 1.2. The usual TEOAE or DPOAE response evaluation is still based on a signal-to-noise relationship, usually in the bands of 2.0, 3.0 and 4.0 kHz. Only the Accuscreen screener (Natus) uses a stochastic model for the TEOAE response evaluation and a spectral coherence model for the DPOAE evaluation [
15,
16,
17,
18]. The algorithms of AABRs are the same as those developed 25 years ago; the AABR algorithm seeks the presence of wave III or wave IV in a predetermined latency range, until a statistical criterion is satisfied [
19].
In terms of terminology, universal neonatal hearing screening (UNHS) is part of an Early Hearing Detection and Intervention (EHDI) program, since hearing intervention and rehabilitation are the top priorities of UNHS [
20,
21]. Different countries have varying approaches and strategies to neonatal hearing screening. In many developed countries, universal newborn hearing screening (UNHS) programs are in place and are considered standard practice [
22,
23,
24,
25]. However, in some low-income countries (Albania, Romania, Bulgaria, etc.), these programs are not yet fully implemented due to limited resources and infrastructure [
26,
27].
Other reports on national neonatal hearing screening programs raise issues around implementation, test procedures, type of tests, coverage, detected cases of hearing loss and costs [
28]. Ensuring high-quality neonatal hearing screening involves maintaining comprehensive coverage (selecting appropriate and accurate screening methods, managing referral rates (rate of failed tests at discharge) effectively and diagnosing hearing disorders as early as possible.
In 2015, Sloot et al. [
29] reported initial data from the European Union project EUScreen on the pediatric vision and hearing screening programs. Regarding the hearing screening, 38 programs participated, and data circulated in EUScreen without being officially published by the corresponding national contributors. The screening information was obtained via the collaboration of professionals from various clinical or university environments. The overall screening paradigm followed three assessment stages, the first two via OAEs and the last one via AABRs or ABRs.
From 2014 to 2019, the International Newborn and Infant Hearing Screening group [
30] asked, via questionnaire, the status of hearing screening practices in 196 countries worldwide; data from 158 countries were obtained and the surprising results show that in 64 countries, there is no organized hearing screening (38% of the world population) and that in 41 countries more than 85% of the babies are screened. For the latter group, the mean living standard was 10 times higher than in countries without screening. In terms of identification times, it was found that the average age at diagnosis of hearing disorders was 4.6 months for screened children and 34.9 months for non-screened children.
While this sort of information (i.e., surveys) is quite valuable for the EDHI national strategies, unfortunately surveys acquiring information directly from individual professionals (as in the case of [
29,
30]) cannot substitute the necessary publications of various national scientific groups on the topic of neonatal hearing screening. In this context, the literature for recent UNHS information suggests a considerable lack of data on EDHI performance and rehabilitation results.
The present paper aims to lessen this informational gap, presenting data on the involvement of OAE-based protocols in the European hearing screening practices as of 2024. The main reason for focusing on the EU data is two-fold: (1) there is already an informational background from the EUScreen project, which needs to be updated; (2) extending the literature update to a world-like scale would exceed the scope of a review paper.
In addition, the paper has sought suitable responses (when information was attainable from the literature) to the following five issues which are very fundamental for EDHI strategies [
29,
31]: (i) Which countries still perform UNHS? (ii) What percentage of newborns are involved? (iii) How many present congenital deafness and more precisely bilateral deafness? (iv) What is the follow-up rate? (v) Which are the most common protocols and OAE technologies used to assess hearing?
2. Materials and Methods
We sourced scientific articles and reviews using the PubMed, Scopus and Google Scholar search engines. Considering that the first papers on UNHS and EDHI started appearing in the literature in the late 90s/early 2000s and since we were interested in the latest advances in hearing screening, we selected a search time-span window of 20 years.
The literature search, conducted on July 2024, followed the PRISMA 2020 guidelines (the PRISMA website was visited in July 2024) and utilized the following keywords (mesh terms): “OAE”, “Universal Neonatal Hearing Screening”, “congenital hearing loss” and “well babies”. Only research articles and review papers were considered good candidates. The standard English language filter was not used, in order to identify information from non-English-speaking, scientific communities and groups. Papers related to UNHS practices outside the EU were not considered. The quality of the material was dictated by several factors, with the publication being in a peer-reviewed journal being the cardinal one. Additionally, clearly stated methodologies and a rigorous application of the established screening protocols were also taken into consideration (i.e., all infants had to be screened for any hearing deficits). The most prominent inquiry results are shown in
Table 1.
The selection criteria for the candidates of the search were based on the following: (i) the origin of the paper (European group or not); (ii) the number of infants screened (large sample studies were preferred); and (iii) the most recent data per country (the most recent study/studies per country were selected).
Exceptions to these rules include the following: (i) cases from similar studies (in terms of sample size) where both manuscripts were included; and (ii) cases where national data and regional data were reported (again both manuscripts were included).
Two independent reviewers went over the papers from Outcome No. 2 (universal newborn hearing screening). There was concordance in the number of the manuscripts selected except one case (for an early Italian screening report of 2006). The final number of eligible papers was 11 (related to 8 UNHS national programs), and they are reported analytically in
Table 2. The PRISMA flowchart process is reported in
Figure 2.
4. Discussion
Deafness can be caused by a variety of factors, which can be broadly categorized into genetic and environmental causes. In fact, deafness can be inherited, often as a result of mutations in specific genes [
50,
51]. These genetic conditions can be autosomal dominant, autosomal recessive or linked to the X chromosome. When hearing loss is associated with other symptoms as part of a syndrome (e.g., Usher syndrome, Waardenburg syndrome) it is called “syndromic deafness”; the most common form of inherited deafness is non-syndromic deafness [
52]. The second causes are environmental ones, such as infections during pregnancy, such as rubella, cytomegalovirus, syphilis and toxoplasmosis. Even factors such as prematurity, low birth weight, lack of oxygen (anoxia) or severe jaundice (hyperbilirubinemia) can lead to hearing loss in newborns. Furthermore, certain medications, particularly some antibiotics (e.g., aminoglycosides) and chemotherapy drugs, postnatal infections and prolonged exposure to loud noise or a sudden loud sound (like an explosion) are environmental causes that can occur after newborn screening. In some cases, the cause of deafness remains unknown (idiopathic), despite thorough investigation.
The first objective of the paper was to provide updates on the European UNHS practices, from reports found in the literature in the period 2004–2024. The European project EUScreen (2015–2019) provided information on 38 UNHS European realities, but the majority of the data were never published analytically in the scientific literature. As such, the EUScreen data are of limited use.
The second objective of the paper was to extract information from the identified on-going UNHS programs regarding the coverage of the project, the congenital hearing impairment estimates (with emphasis into the bilateral hearing loss), the description of causes leading to hearing loss and the relative intervention strategies and lastly the technologies and protocols used.
4.1. The Number of UNHS Programs
The first impression from the database queries was that the number of papers related to the topic of otoacoustic emissions and hearing screening was relatively low. The data in
Table 1 (see the shaded columns) suggest that in 2024, the topic of hearing screening is not highly considered, despite the fact that considerable amounts of information are missing from the literature.
The data from Sloot et al. [
29] have indicated that the number of collaborating UNHS programs in the EU was 38. Analytical reports in the literature (2004–2024) were found from only eight screening realities. In the initial search, a number of papers describing small (local or pilot programs) were found, but they were not included in the review, since the data were referring to small samples. It is not clear at this point if (1) the unofficial reports of the EUScreen project (found at
www.euroscreen.org/reports (accessed on 25 September 2024)) constitute some sort of official record in the minds of the European Audiologists and researchers; or (2) some data have been published in local European journals outside the indexing of the Pubmed and Scopus databases.
4.2. Reported UNHS Coverage
The reported coverage estimates are quite high (i.e., >90%), but the data primarily report specific years and specific regions. Only the Polish data correspond to a wide-spread national hearing screening program.
There are some conflicts between the coverage values reported by Sloot et al. [
29] (see
Table 2, column 5) and the ones from the reported data in the literature. Important differences can be seen in the data from Albania (10% vs. 23%, or 96.6%), France (50% vs. 99%), Germany (95% vs. 50%) and Italy (70% vs. 94% or 93.8%). It is quite possible that these differences are generated by the fact that none of the reported data refer to a national estimate, but instead reflect data from different local areas and possibly from different times (the Sloot et al. EUScreen data were published in 2015, which means that for the majority of programs, the coverage estimates were from 2014 or much earlier).
4.3. Estimates of Bilateral Hearing Loss Prevalence
For the majority of programs, the estimates of bilateral hearing loss refer to the average number, of well babies and intensive care unit infants with hearing loss, without considering the discrete diversities between well babies and NICU residents.
These estimates concord with the data trends reported in the literature for the UNHS realities outside Europe [
24,
25,
53,
54,
55,
56].
The prevalence in Switzerland [
46] and in the Italian Umbria NICU sample [
40] present the highest values, with 20.94 and 41 infants per 1000. For the first group, the prevalence refers to a small sample from the area of Zurich, and as such, the national estimate should be different. The Metzger et al. paper [
46] does not include detailed information on the loss-to-follow-up estimate, nor any data on possible demographic factors which might influence the final prevalence. The same rationale can be applied to the Molini et al. paper [
40].
4.4. Causes Leading to Hearing Loss and Intervention Strategies
Very few papers have examined the causes of congenital hearing loss presented by the screened WB and NICU infants. The only project which provided etiological data is the Umbria UNHS project, where factors such as non-syndromic genetic mutations with familiarity, neonatal intensive care stay, cytomegalovirus in utero infection, genetic and syndromic factors, craniofacial anomalies and neurodegenerative disorders were reported.
The intervention policies included unilateral and binaural hearing aids and cochlear implants. The exact times of the cochlear implant surgery are not always reported; the time-estimates reported refer to times > 15 months of age (Polish UNHS program).
The data from the Russian UNHS program are quite interesting in the fact that they show that NHS practices do not always capture infants presenting genetic complications [
43,
44]. This can be explained by the fact that these complications have a relatively slow onset (and impact on cochlear functionality), which does not always coincide with the first days of life. The Russian data suggest that for a completer and more accurate UNHS program, a genetic screening of the most frequent mutations should be conducted as well, at least in the NICU population.
4.5. Technological Issues
All of the identified UNHS programs used a two-stage protocol (TEOAE + TEOAE) where the third stage was an ABBR or directly a diagnostic ABR evaluation. Distortion product otoacoustic emissions were not utilized. There is a lack of information on the actual TEOAE hardware used, but the descriptive information of these projects refers primarily to portable TEOAE screeners, which have the option to carry out an AABR assessment on the tested infant.
Previous data in the literature [
57,
58] have identified and recommended the need to have a consensus on the characteristics of the TEOAE probes across the various manufacture models, which might alter the screening outcomes, especially in borderline screening scenarios. Unfortunately, this aspect was missed completely in all of the UNHS reports presented in this review.
4.6. Some Comments About the Search Engines Using Artificial Intelligence (AI) Interfaces
With the introduction of artificial intelligence tools, the Scopus database offers new possibilities of research, exceeding the standard paradigms of Mesh terms and Boolean operators.
Unfortunately, this approach seems to have a number of limitations. In fact, although useful for many general questions, AI cannot replace the human operator and cannot be used as the sole source of information, as it does not really understand the meaning of words or phrases but relies on statistical models to generate the answers. As an example, we report the answer to a specific question about the aim of this review, where the references quoted are analyzed but the data included are only partial. The question “Which are the European countries where a Universal Neonatal Hearing Screening is active?” was submitted to the SCOPUS AI. The AI tool responded: “Based on the abstracts reported, the countries where Universal Neonatal Hearing Screening (UNHS) is carried out include Poland, France and Switzerland. However, it’s important to note that the abstracts do not provide a comprehensive list of all countries where UNHS is carried out. The information is limited to the countries mentioned in the abstracts, and there may be other countries implementing UNHS that are not covered in the provided abstracts”.
The Scopus AI tool identified 3 out of the 11 papers used in this review. Such a tool can be used as an initial guideline, but obviously needs to become more precise and examine the total lexical content of a manuscript. It should be also mentioned that numerous publications in the literature refer to a specific condition where Artificial Intelligence bots produce fictitious and erroneous information, which is called AI hallucinations [
59,
60,
61]. In this context, the use of AI bots to extract information needs to be constantly monitored till the produced errors are minimal.
4.7. Limitations of the Study
The results obtained from the database queries refer to regional (not national) studies with large datasets (i.e., >1000 subjects) in order to attain a certain solidity of the reported results. A number of studies with significantly smaller sample sizes were also identified but were not included in the body of manuscripts of this review. It might have been more informative to include this sort of study, but all of the contributors to this review were against it.
Another negative aspect of systematic reviews is the fact that they depend on the proper indexing of the databases used. In this context, we might have lost a number of recent papers because they were not properly indexed in Pubmed, Scopus and Google Scholar.
We are also including a remark from an anonymous reviewer who suggested that many European clinical realities might use dedicated AABR/ABR protocols to assess the neonatal population at hand. In this context, the PRISMA review conducted would miss a number of publications where the terms OAE or UNHS were not included. On the other hand, according to the EUScreen practices ALL regional or national programs use a three-phase UNHS protocol, involving OAEs in the first screening stages; therefore, the information not included in this review would reflect the screening outcomes only from small clinical realities.