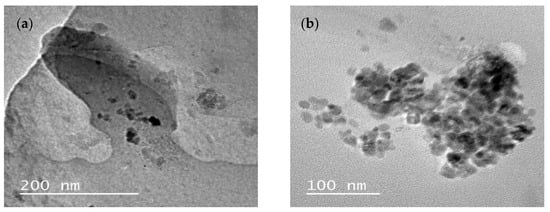
2.2.1. Particle Size and Polydispersity Index
In this study, the nanoemulsions of NanoEE-Globulus showed smaller droplet sizes when compared to the nanoemulsions of NanoEE-Citriodora. Both nanoemulsions showed physicochemical stability concerning the parameters of droplet size and polydispersity index, as shown in
Table 2.
In this study, the samples of NanoEE-Globulus and NanoEE-Citriodora showed a mean size around 100 nm, with PDI values close to 0.3. The size of the nanoemulsion droplets depends on factors such as the nature and concentration of the surfactant in the aqueous and oil phases, the nature of the essential oil being used, the solvent polarity, and the nature and the proportion of the aqueous and oil phases [
21]. Small droplet diameters, ranging from 100 to 200 nm, as in the results found in the present study, are fundamental for the absorption and in vivo distribution of suspensions [
23]. In addition, when considering the development of a nanoemulsion for therapeutic purposes, the determining points for the release of the active agent are droplet size and uniformity [
24].
The larger the PDI, the larger the particle size range and, consequently, the more heterogeneous the droplet size is in the nanoemulsion [
25]. PDI results below 0.3 (as in the present study) show a narrow distribution of size and good homogeneity of the nanoemulsion [
22]. The lower the value found for PDI ≤ 0.2, the higher the degree of homogeneity in droplet size, which is an important fact when considering the possibility of the future development of mouthwash because if the system remains with homogeneous droplet size, the product is likely to have greater physicochemical stability [
22]. The low values obtained for span corroborate the PDI values, showing a narrow particle size distribution.
2.2.2. Zeta Potential and pH
In the present study, the nanoemulsions of NanoEE-Globulus and NanoEE-Citriodora have zeta potential values ranging from −19 to −30 mV and pH values close to 7, tending to be neutral. The surfactant polysorbate 80 has an apolar part that interacts with the oil and a polar part outside the nanoemulsion, which exposes the negative charge to the nanoemulsion, resulting in a negative surface density, owing to the presence of oxygen atoms in the molecule [
22].
The zeta potential measurements depend mainly on the stabilizing agents and the pH of the medium [
26]. The zeta potential reflects the surface load of the nanoemulsions and indicates the stability of a nanoemulsion, according to the repulsive forces between the droplets and the potential changes on the droplet surface [
27]. Also, it evaluates the capacity that particles can remain in suspension, that is, without aggregation or sedimentation [
28].
In general, the zeta potential evaluates nanoemulsion stability; when using blocks of anionic surfactants, such as polysorbate 80 (Tween 80) and sorbitan monooleate (Span 80), as in the present study, dispersion stability is verified by steric impediment, avoiding the coalescence of particles and allowing physicochemical stability [
29]. In addition, the absence of electrostatic repulsion is also confirmed by the pH results, with a tendency to be neutral [
30]. When there is additional steric stabilization (Tween 80, in this case), values around 20 mV are already sufficient to maintain the stability of the colloidal system [
27,
31].
2.2.4. Antimicrobial Activity Tests: Inhibitory Concentration of Nanoemulsions
The broth microdilution test was used to determine the MIC of the nanoemulsions. Turbidity was the parameter used to verify the growth of the microorganism in the microplate wells. To this end, the MIC was the lowest concentration among those tested, and there was no turbidity in the growth medium. Both nanoemulsions, NanoEE-Globulus and NanoEE-Citriodora, were effective in controlling
S. mutans (
Table 3). The sample of NanoEE-Globulus presented an MIC of 4% while that of NanoEE-Citriodora showed an MIC of 6% against this Gram-positive bacterium, revealing that the samples present inhibitory activity even at the lowest concentration tested.
The antimicrobial action mechanisms of eucalyptus essential oil consist of denaturation of the action of bacterial proteins, inactivation of microbial enzymes, alteration of membrane permeability of Gram-negative bacteria, and chelation of cation ions present in bacterial cytoplasm [
35]. In a study evaluating antimicrobial action, eucalyptus essential oil demonstrated effectiveness against bacteria present in the oral cavity
(S. aureus and
E. faecalis), with efficacy similar to that of 0.12% chlorhexidine, which is considered the gold standard for mouthwashes [
36].
Harkat Madouri et al. [
37] conducted a literature review that evaluated the effect of
Eucalyptus globulus labill essential oil on microorganisms related to periodontal disease and reported that the following MICs are, respectively, needed to inhibit the bacteria
P. gingivalis (0.28 mg mL
−1),
F. nucleatum (1.14 mg mL
−1), and
A. actinomycetemprincipans (AA) (9.13 mg mL
−1). The result highlights the highest resistance of AA.
There is a limitation in the literature when comparing the results of the present study; in addition to the limited number of studies that have tested essential oils for application in mouthwashes, there is a diversity of presentations, methodologies, and oils. There is a predominance of in vitro studies in particular [
18,
22,
38,
39]; one study studied the in vitro and in vivo phases in animals [
40], and one was a randomized double-blind clinical trial [
14].
In a recent study, Karnjana et al. [
41] found that the ethanolic extracts of
Streblus asper,
Cymbopogon citratus, and
Syzygium aromaticume can be natural agents with multiple actions on
S. mutans. Changes in the bacterial cell walls occurred after treatment with the ethanol extracts, increasing hydrophobia and decreasing the formation of bacterial biofilms for 24 h. The authors reported that the extracts were also used for the green synthesis of silver nanoparticles, and the results were also satisfactory.
2.2.5. Nanoemulsion Cytotoxicity and Cell Viability Assays
The results for cell viability indicated that nanoemulsions of NanoEE-Globulus showed higher cell viability compared to the NanoEE-Citriodora samples. Specifically, the nanoemulsions at the concentration of 100% maintained a cell viability greater than 50%, suggesting that these nanoemulsions do not have a significant cytotoxic potential (
Figure 2). This aspect is essential for using them in products such as mouthwashes.
The difference in cell viability between the nanoemulsions of the NanoEE-Globulus and NanoEE-Citriodora samples can be attributed to the distinct chemical properties of the essential oils involved. Eucalyptus globulus contains 1,8-cineole, a compound that has been associated with a relatively high safety profile for cells at high concentrations. This may explain the result for higher cellular viability, suggesting that NanoEE-Globulus is less toxic to cells.
In contrast, the major component of Eucalyptus citriodora is citronellal, which despite its effective antimicrobial properties, may have a more pronounced impact on cell viability, resulting in lower viability compared to NanoEE-Globulus.
The observation that nanoemulsions, even at a concentration of 100%, maintained a cell viability of more than 50% is particularly relevant for applications in products such as mouthwashes. The absence of a cytotoxic potential at high concentrations is a positive point, as it ensures that the product is safe for prolonged use and in direct contact with oral tissues.
Cellular safety is an important factor for the formulation of oral care products because the combination of antimicrobial efficacy and low toxicity is essential. Nanoemulsions of NanoEE-globulus, with their higher cell viability and absence of cytotoxicity at high concentrations, offer a promising profile to be used in mouthwashes and other oral care products. On the other hand, formulations containing NanoEE-Citriodora may need adjustments in their concentration or formulation to ensure similar safety, which is a point to be considered for future applications and product development.
2.2.6. Antimicrobial Activity Assays: Inhibitory Concentration of Mouthwashes Functionalized with Nanoemulsions
Fluoride mouthwashes showed a bacteriostatic effect against
S. mutans in all study concentrations, while fluoride-free mouthwashes showed this effect in concentrations above 8%, as shown in
Figure 3.
The antimicrobial action of nanoemulsions at different concentrations was confirmed by comparing them with the control sample containing only the mouthwash. In addition to the bacteriostatic effect, the bactericidal effect was also confirmed in samples that did not present turbidity. The formulation of oral mouthwashes has compounds such as sodium fluoride, which have antimicrobial activity to eliminate harmful oral microorganisms, thus contributing to the prevention of future dental lesions, gingivitis, and periodontitis.
In this study, eucalyptus nanoemulsions demonstrated a synergistic effect with sodium fluoride. When applied topically, such as in toothpastes, gels, or fluoridated solutions, fluoride provides direct and immediate protection [
42]. Fluoride efficacy is even greater when combined with good oral hygiene, which includes regular toothbrushing, flossing, and periodic visits to the dentist. This ensures that teeth are less susceptible to the development of cavities and other oral diseases. The mechanism of action against fluoride is mainly associated with its influence on the mineralization of teeth and the process of remineralization, in addition to its impact on plaque bacteria, which cause acidification and demineralization [
43].
Fluoride can affect bacterial metabolism in several ways. One of them is acting directly as an enzyme inhibitor, for example, inhibiting the glycolytic enolase enzyme. Another form of action involves the formation of metal–fluoride complexes, such as AlF
4−, which are responsible for inhibiting F-ATPases, which are enzymes that translocate protons. These complexes mimic phosphate, forming complexes with ADP in enzyme reaction centers [
43].
However, the most relevant actions of fluoride for reducing plaque cariogenicity are related to its weak acid character. Fluoride increases the permeability of the bacterial membrane to protons, compromising the functioning of F-ATPases in proton export. This leads to cytoplasmic acidification and inhibition of glycolytic enzymes, reducing acid tolerance in bacteria. Fluoride is particularly effective in acidic environments; for example, in acidic conditions in the cariogenic plaque, concentrations as low as 0.1 mm of fluoride can completely stop glycolysis in intact
Streptococcus mutans cells [
44].
In general, the anticaries actions of fluoride are complex, involving effects on both the bacteria and mineral phases of plaque formation. Its antibacterial properties are complex but predominantly influenced by its weak acid character [
44].
In synergy with fluoride, eucalyptus essential oil also presents an antimicrobial action. The antimicrobial action of eucalyptus essential oil can be attributed to different compounds, which vary according to the species of eucalyptus and the cultivar. In the case of the species
Eucalyptus globulus, the main compound responsible for antimicrobial activity is 1,8-cineole (also known as eucalyptol). Studies such as that of Goldbeck [
45] indicate that 1,8-cineole can represent up to 71% of essential oil, while other studies, such as the one of Salem (2018) [
46], mention a concentration of approximately 13.23%. This variation can be the result of differences in the origin of the samples or in the methods of analysis.
For the species Eucalyptus citriodora, the main compound is citronellal, which can account for to 72.7% of the essential oil. Citronellal has antimicrobial properties and is also known for its insect repellent effect. Therefore, the antimicrobial efficacy of eucalyptus essential oil can depend greatly on the specific profile of the compounds present, which varies with species and cultivar.
The study carried out by Choi [
47] showed that essential oils containing aldehydes as major compounds exhibit considerable antimicrobial activity against strains of
S. mutans. The work of Goldbeck et al. [
45] showed that the essential oil of
E. globulus with a high concentration of 1–8 cineole caused the microbial death of strains of
S. mutans. According to the authors, the antibacterial activity of these compounds is linked to an increase in the permeability of the bacterial membrane and the consequent loss of its cellular elements, which leads to cell collapse.
In the future, mouthwashes are expected to advance to the point of being highly specialized, with action against specific pathogenic bacteria, preventing other beneficial bacteria from the oral microbiome from being affected, thus promoting the balance of such microbiome. In addition, they could modulate the immune response of the host, helping the body fight infections more effectively and even preventing inflammation. These advances would bring significant benefits to oral health, especially for people who have difficulty performing adequate oral hygiene or for those with medical conditions that affect oral health.