3.1. Kinetic Experiments
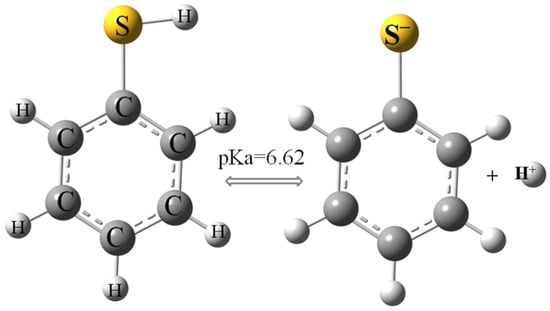
The evolution of thiophenol removal on AmberLite®IRA402 over time (kinetic experiments) was performed at different pH levels and ionic strengths of the solution. Figure 3 shows that both these physicochemical parameters strongly influenced the sorption process. The resin used in this work was a strong base anion Type I resin containing quaternary amine groups. It allows anions to be effectively removed from water, including from organic compounds. Studies using IRA 402 resin show the possibilities of the removal from the aqueous environment of even large anionic structures, such as sulfonated azo dyes: brilliant yellow [53] and Acid Blue 113 [54]. Thiophenol, depending on the pH of the aqueous solution, may also occur as an anion (Figure 1).
As can be seen in Figure 3, the sorption efficiency was actually highest when thiophenol was present mainly or completely in anionic form (pH 7 and 9) in an aqueous solution. As can be seen in Figure 3, the results of the experiment indicate that the most effective sorption was observed for thiophenol solutions prepared in distilled water. The addition of NaCl caused chloride ions to appear in the solution that definitely competed with thiophenolate ions. Therefore, the sorption of thiophenol from saline waters (ionic strength 0.1 M) was much less efficient.
The contact time significantly influences the sorption capacity of sorbent. As can be seen in Figure 3, the equilibrium state was established very quickly, after just 20 min of phase contact time at pH 7 and 9. In the case of sorption in an acidic environment (pH 4), the process was much slower and took about 80–90 min. The equilibrium state was established during the sorption of thiophenol on sepiolite for a longer period and lasted about 100 min [14]. On the other hand, the time required to reach sorption equilibrium for Acid Blue 113 on Amberlite IRA 402 was 90 min [54].
One possible way of simulating the sorption processes that is often used in predicting water treatment processes is sorption reaction models. Pseudo-first-order and second-order models are widely used models of sorption reactions and consist of fitting appropriate kinetic equations (Table 2) to experimental data without delving into the sorption mechanism. The parameters of the kinetic equations were determined with the nonlinear least-squares method using the Levenberg–Marquardt algorithm (the miner function in Mathcad). The results of this fitting are presented in Figure 4 and Table 2. The RMSE index (root mean squared error) and Chi2 (Pearson’s Chi-squared test) indicate the quality of the fit.
As can be seen in Table 2 and Figure 4, both models reflect the experimental results well. In experimental conditions that are closer to natural conditions (thiophenol solution with NaCl), the pseudo-second-order model gives a better fit. The theoretical values of sorption capacity (qe) are very close to the results of the experiments. These calculated values are 0.44, 2.93 and 2.10 mg/g for pH 4, 7 and 9, respectively (Table 2). The corresponding experimental values resulting from the trend of qe versus the sorption time (Figure 3 and Figure 4) are 0.42, 2.91 and 2.10 mg/g for pH 4, 7 and 9, respectively. The sorption process of thiophenol solution in distilled water at pH 7 also very well reflects the pseudo-second-order reaction model. The theoretical and experimental sorption capacity is 14.97 and 14.66 mg/g, respectively. The literature data indicate that in the case of sorption of organic anions onto strongly basic anion exchange resins, pseudo-second-order kinetic models usually describe experimental data better than pseudo-first-order models [55,56,57,58]. This trend includes both large anionic dye molecules [55,57] and small molecules structurally similar to thiophenol, such as phenol [56,58].
On the other hand, the kinetic trend of the sorption process of thiophenol solution in distilled water at pH 4 fits the pseudo-first-order reaction model slightly better. The theoretical sorption capacity in this condition equals 0.46 mg/g (exp. 0.44 mg/g). At pH 9 for thiophenol solution prepared from distilled water, it is problematic to determine which model actually fits the experimental data better. The RMSE index indicates that the pseudo-first-order is slightly better, but the Chi2 index indicates that the pseudo-second-order is slightly better (Table 2). At pH 9, the theoretical sorption capacity is 14.09 mg/g for pseudo-first-order estimation, and 14.79 mg/g for pseudo-second-order estimation (exp. 14.27 mg/g).
3.2. Sorption Experiments
Figure 5 shows the amount of thiophenol absorbed by an IRA402, expressed as a function of the equilibrium concentration of sorbate at a room temperature. As can be seen in Figure 5, the experimental results in static conditions are consistent with those obtained in the kinetic experiment. The sorption capacity of IRA402 is highest during the sorption of thiophenol from a distilled water solution at pH 7 and 9: 13.83 and 13.95 mg/g, respectively. The sorption capacity significantly decreases to values of 2.83 mg/g (pH 7) and 2.48 mg/g (pH 9) when the ionic strength of the solution increases. Sorption in the acidic condition occurs to a very small extent. In this case, there is basically no difference due to the ionic strength of the solution. The sorption capacity of thiophenol at a concentration of 30 mg/L from the solution prepared using distilled water is 0.37 mg/g, and from the solution containing sodium chloride (ionic strength 0.1 M), it is 0.42 mg/g.
The sorption equilibrium results showing the dependence of the amount of adsorbed substance on the concentration of the adsorbate at a constant temperature (Figure 5) were fitted to the Langmuir and Freundlich isotherm models [59,60]. The study of sorption isotherms is important from the point of view of the design and operation of sorption systems [61]. The parameters of the isotherm equations were determined in the same way as in the kinetic experiments by using the nonlinear least-squares method and the Levenberg–Marquardt algorithm. The results of this fitting are presented in Figure 5 and Table 3.
The sorption equilibrium results that show the dependence of the amount of adsorbed substance on the concentration of the adsorbate at a constant temperature (Figure 4) were fitted to the Langmuir and Freundlich two isotherm models [59,60]. The study of sorption isotherms is important from the point of view of design and operation of sorption systems [61]. The parameters of the isotherm equations were determined in the same way as in the kinetic experiments using the nonlinear least-squares method and the Levenberg–Marquardt algorithm. The results of this fitting are presented in Figure 6 and Table 3.
Analysis of the estimated values of the fitting indexes of the theoretical equations (RMSE, Chi2) (Table 3) shows that the process of thiophenol sorption from the solution is better described by the Langmuir isotherm in each case. According to the literature, similar results have been reported for the sorption of other organic sorbates on strong base anion exchange resin. The Langmuir isotherm better fits the experimental results during the sorption of Acid Blue 113 dye on Amberlite IRA 402 [54], Acid Orange 7, Acid Orange 10 and Acid Red 88 dyes on Amberlite IRA-458 [62] and phenol on Amberlyst A26 [63].
The estimated values of the QL parameters (maximum sorption capacity) from the Langmuir equations are in good agreement with the results of the experiment and in all cases slightly higher than the experimental values. For the thiophenol solutions prepared using distilled water, they are 0.571 mg/g for pH 4 (exp. 0.37 mg/g), 18.936 mg/g for pH 7 (exp. 13.83 mg/g) and 16.351 mg/g for pH 9 (exp. 13.95 mg/g). For the thiophenol solutions with 0.1 M ionic strength (with addition of NaCl), they are 0.734 mg/g for pH 4 (exp. 0.42 mg/g), 4.853 mg/g for pH 7 (exp. 2.83 mg/g) and 4.684 mg/g for pH 9 (exp. 2.48 mg/g).
3.3. Desorption Process of Thiophenol from AmberLite®IRA402 Resin
One of the key points of the planned industrial use of ion exchange resin is its effective regeneration and readiness for reuse, so that the installation can be economically viable and environmentally friendly [64]. The recycling of used resins is a problem studied primarily on a laboratory scale. During experiments, the appropriate washing solution was selected so that the effects of the desorption process was as high as possible. The suppliers of Amberlite®IRA402Cl resin present different regenerants for their products in the product data sheet, for example Lenntech [65] and BSBL [66] propose NaOH solution (2–4%), and Dupont [67] proposes regeneration with solutions, where the main component is 10% NaCl (in the composition also HCl, NaOH and H2SO4 are listed). In turn, Marin and Stanculescu carried out desorption of acid blue 113 previously adsorbed on Amberlite®IRA402 using hydrochloric acid (molar concentration of HCl 1.0–7.0 M) [54].
Due to the discrepancies in the used regeneration solutions, in this study, we conducted a sorption experiment of thiophenol at pH 7. Sorption was the most effective in that condition. The sorption process was performed using 0.2 g of resin and thiophenol solution with a concentration of 30 ppm. Then, the process of desorption of thiophenol under the influence of various reagents was performed. The reagents used were distilled water, HCl (4%, 9%, 18%), 10% NaCl and 4% NaOH. Figure 7 shows the influence of the regenerant on the desorption of thiophenol from the Amberlite®IRA402 resin. The thiophenol desorption expressed the ratio of the mass of thiophenol in the solution after the desorption experiment (with different regenerants) to that mass of thiophenol which was sorbed during sorption (in a percentage). As can be seen in Figure 7, the type of regenerant was very important in this case to obtain the best regeneration results. In our experiment, when applying the desorption test, the amount of thiophenol in solution above the resin was the highest (ca. 90%) in the case of hydrochloric acid. This means that at pH 7 the ion exchange process was mainly responsible for the removal of the thiophenol from the solution. During 90 min of shaking with 10% NaCl solution, practically no desorption of thiophenol from the resin was observed. This means that thiophenol was strongly bound to it. Paulino and Afonso also observed that strong basic anion-exchange resins strongly retained sulphur compounds (with S2− ions) [68]. It can be also seen that the desorption of thiophenol did not change with increasing HCl concentrations from 4 to 18%, which indicates that at pH 7 ion exchange is not the only mechanism for removing thiophenol from an aqueous solution and a small part of it is adsorbed on the resin surface.
3.4. Investigation of the Sorption Process of Thiophenol on AmberLite®IRA402 Using FT-IR Spectroscopy
Various vibrational spectroscopy techniques, such as FT-IR spectroscopy, are often used to obtain valuable information concerning the sorption process. The analysis of changes in the infrared spectrum of sorbent during this process is very frequently helpful in understanding it. IR studies enable the identification of functional groups which are characteristic of pure sorbate and sorbent and which indicate the participation of these specific functional groups in sorption interaction after sorption. The use of infrared spectroscopy has been described in the literature, to both characterize IRA 402 resin and to confirm that the surface of this resin has been modified by various chemical syntheses [63,69,70,71]. Infrared spectroscopy has also been used to characterize possible interactions between the resin and the adsorbed substance [54,71]. The analysis of experimental and theoretical vibrational spectra (IR and Raman) of the sorbate (thiophenol) has been described many times. Authors have most often described the spectrum of thiophenol in undissociated form and compared it with the spectra of the obtained complexes with noble metals (Ag and Au) [72,73,74,75,76]. This ability to bind to metals makes thiols useful as selective sorbents for removing heavy metals from the aqueous environment [77,78].
Figure 8 shows a comparison of the FT-IR spectra of thiophenol, pure ion exchange resin IRA 402 and IRA 402 after the sorption of thiophenol (at pH 4, 7 and 9). Infrared spectroscopy studies confirm that IRA 402 resin adsorbs very small amounts of thiophenol in acidic media. This is evidenced by very small changes in the spectrum of the resin, which was in contact with thiophenol for 2 h at pH 4. The most intense bands originating from thiophenol at 1583, 1480, 1442, 735 and 688 cm−1 slightly influenced the shape of the bands in the infrared spectrum of the resin after contact with thiophenol.
Changes are evident in the infrared spectrum of IRA 402 resin after the sorption process at pH 7 and 9 (Figure 8). In addition, they clearly indicate that thiophenol was adsorbed in anionic form in these conditions. The presence of the thiophenolate ion is manifested by a shift of the band at 1583 cm−1 to 1574 cm−1. This IR signal is associated with the C-C stretching vibrations in the benzene ring and appears in the dissociated form of thiophenol at a frequency of ca. 1575 cm−1 [76,79,80]. Figure 8 shows that the band at 1092 cm−1 (thiophenol) shifts to lower wavenumbers to 1083 cm−1 (thiophenolate adsorbed on IRA 402 at pH 7 and 9). This band mainly corresponds to C-S stretching vibration [73,76]. Significant changes in the spectra are also observed around 700 cm−1. The intense bands at 735, 697 and 689 cm−1 seen in the spectrum of undissociated thiophenol are not observed after the thiophenol sorption process on the ion exchange resin (pH 7 and 9, Figure 8). These bands come from C-H out-of-plane bending vibrations (735 cm−1), ring in-plane deformations (699 cm−1) and ring out-of-plane deformations (689 cm−1) [77]. However, in the experimental spectrum of resin IRA402 with adsorbed thiophenol (pH 7 and 9) strong bands at 741 and 697 cm−1 are observed. Additionally, a weak band appears at 1256 cm−1, which is most likely due to changes upon sorption in the vibrations associated with the amine functional group of the resin. In the pure resin Amberlite@IRA402, the bands in this spectral range (at 1268 and 1243 cm−1) are connected mainly to the stretching vibration of the C-N bond and the C-H bending vibration in –CH2− group between the ring and the N(CH3)3 group [81]. The second explanation for the appearance of the band at 1256 cm−1 could be the change in the C-H vibrations in the aliphatic chain of the resin [80].
The obtained theoretical (DFT/PCM/B3LYP/6-31g**) infrared spectra for dissociated and undissociated thiophenol structure (Figure 9) are consistent with the results of the experiment. It should be noted that, to the best of our knowledge, calculations of the infrared spectra of thiophenol and thiophenolate ion using a solvation model (PCM) have not yet been reported in the literature. The strong signal associated with the C-C stretching vibrations in the benzene ring seen in the theoretical spectrum of thiophenol at 1607 cm−1 also shifts towards lower frequencies and appears in the calculated spectrum of the dissociated form of thiophenol at 1596 cm−1 (experimental counterparts are at 1583 and 1574 cm−1, respectively, Figure 9). The mixed vibration originating from C-H in-plane bending (in the ring) and C-C stretching is seen in the theoretical spectrum of the thiophenol and thiophenolate anion at 1490 and 1473 cm−1, respectively. The strong intensity band that mainly originates from C-S stretching vibration also shifts towards lower wavenumbers. This is observed for thiophenol at 1091 cm−1 and for the thiophenolate anion at 1075 cm−1 (experimental counterparts are at 1092 and 1083 cm−1, respectively, Figure 9).
The strong bands in the experimental spectrum of thiophenol in the range of 750–680 cm−1 (at 735 and 688 cm−1 (699 cm−1 shoulder)) are present in the theoretical spectrum at 737 and 690 cm−1 (689 cm−1 shoulder). In the case of the anionic form of thiophenol, these bands lose intensity but only slightly change their positions (Figure 9). Therefore, in the spectrum of the thiophenolate anion adsorbed on the resin at pH 7 and 9 (Figure 8), there should be no change in the frequency of the bands in this spectral region relative to the spectrum of pure undissociated thiophenol. However, the changes are clearly visible and cannot be explained solely by the presence of thiophenol as the thiophenolate ion. As mentioned earlier, strong bands at 741 and 697 cm−1 are observed in these spectra (pH 7 and 9, Figure 8). This indicates that in-plane ring deformation, out-of-plane ring deformation and out-of-plane C-H bending vibrations of thiophenol do not vibrate completely freely after the sorption process. This finding indicates that—together with the ion exchange process that occurs between the dissociated thiol group and the quaternary ammonium group—the interaction (van der Waals and/or π-π) between the aromatic structures of thiophenolate anion and IRA402 also takes place.
Source link
Katarzyna Chruszcz-Lipska www.mdpi.com