By virtue of the high reactivity of plant polyphenols, which can endow the target polymeric materials with favorable physicochemical properties via direct blending, polymerization, coordination, and modification followed by polymerization. Importantly, the unique features of plant polyphenols can also be effectively strengthened or mitigated through material engineering technology [
55]. Within this review, some attempts about fabricating plant polyphenol-derived hybrid materials in recent years have been systematically summarized. Particularly, plant polyphenols-metal or/and polymeric materials are discussed in detail, focusing on their chemical architectures, product features, and possible applications in fields such as resistance to fire, water-treatment, functional leather, stimuli-responsive materials, biomaterials, sensing materials, etc.
3.2. Materials Based on Epoxy Resin
As one of the most representative thermosetting polymers, epoxy resins possess the desired mechanical and thermal properties, good chemical resistance, as well as ideal processability [
80,
81,
82], on the basis of an adjustable internal crosslinking network formed of different kinds of epoxy monomers and curing agents [
83] Consequently, epoxy resins have been widely used in various fields, such as coatings, adhesives, constructions, etc. [
84,
85,
86,
87,
88]. In recent years, biobased epoxy resins have received increasing attention from researchers. Interestingly, renewable and sustainable plant polyphenols can be used to replace the toxic monomer (such as bisphenol A) in the synthesis of epoxy resins. For instance, Benyahya et al. proposed a new method for the synthesis of biobased epoxy polymers based on green tea polyphenols. Firstly, the green tea polyphenols were modified by epichlorohydrin to yield the epoxy pre-polymers (GEGTE). Subsequently, the GEGTE pre-polymers were combined with isophorone diamine (IPD) to create the polyphenol-derived epoxy resin (GEGTE-IPD), which possessed higher crosslinking density and thermal stability. In addition, compared with fossil-based epoxy polymers composed of bisphenol A and IPD, GEGTE-IPD exhibited a higher glass transition temperature and better mechanical properties [
89]. Similarly, Long et al. fabricated a polyphenol-based epoxy resin monomer (pyrogallol triglycidyl ether) from biological pyrogallol by combining epichlorohydrin with olefin epoxidation, which can be further cured using methyltetrahydrophthalic anhydride. Importantly, the target epoxy resin shows ideal thermomechanical properties (i.e., glass transition temperature of 164 °C, storage modulus of 3500 MPa) [
90]. The above-mentioned studies demonstrate that the addition of plant polyphenols can effectively improve the thermal and mechanical properties of the resulting epoxy resins, providing new methods for creating polyphenol-derived epoxy resins. Meanwhile, the green preparation concept of epoxy resins will also receive increasing attention.
In addition, plant polyphenols can also be used as biobased flame retardants and hardeners to prepare high-performance epoxy resins. In work reported by Esmaelli et al., a novel epoxidized tannic acid (ETA) foam was designed and fabricated. Specifically, tannic acid was first epoxidized via glycidylation and then participated in the subsequent foaming and curing processes to obtain the target ETA foam with a yield of 98% (
Figure 7a). The ETA foam showed relatively lower thermal conductivity (0.0286 W m
−1 K
−1), enhanced char yield (48.3% in air), and a higher limiting oxygen index (LOI) value (36.8%), which can be regarded as a self-extinguishing material [
91]. Similarly, Kim et al. fabricated a tannic acid-based thermosetting epoxy resin, in which tannic acid served as both a flame retardant and a renewable hardener (
Figure 7b). In this work, the target biobased epoxy resin exhibited the desired thermal and mechanical properties (i.e., glass transition temperature of 155.4 °C, Young’s modulus of 2900 MPa), as displayed in
Figure 7c–e. In addition, with an increase in tannic acid amounts, the resulting resins showed improved flame-retardant properties (
Figure 7f), as the tannic acid can act as an oxygen free radical quenching agent and a carbonization agent [
92]. In addition, Feng et al. reported the synthesis of a biobased thermosetting epoxy resin, in which tannic acid with diverse bonding capacities and unique branched structure can be regarded as a multifunctional curing agent (
Figure 7g). The results demonstrated that it is possible to prepare epoxy resin with high mechanical properties (tensile strength of 98.4 MPa) and tunable functionalities, including damping, recyclability (based on the transesterification process), and shape memory (based on the entropic elasticity) [
93]. The above design concepts may provide new and promising insights for the fabrication of biobased high-performance multifunctional epoxy polymers.
In summary, different kinds of plant polyphenols have been employed to construct environmentally friendly epoxy monomers and resins. However, it should be mentioned that some plant polyphenols derived from different resources usually need to be modified or functionalized based on their active Ph-OH groups for further curation. Limited by the varied reactivity and complicated structure of plant polyphenols, the synthesis processes of biobased epoxy resins may not be easily reproducible in industry and difficult to apply in fine chemicals. To this end, some attempts are being made to depolymerize complex plant polyphenols and use relatively low-molecular-weight monomers for creating epoxy derivatives. Based on these green synthetic techniques, more and more fully biobased epoxy resins from plant polyphenols will emerge and be applied in different fields.
3.3. Materials Based on Phenol-Aldehyde Polymer
The combination between phenols and aldehydes has been broadly explored at an industrial level to achieve the preparation of coatings, adhesives, foams, and composites [
94,
95]. In the synthesis of phenol-aldehyde polymers, biobased plant polyphenols can be used to improve the physicochemical properties of the resins. For example, due to the good affinity of plant polyphenols to aldehydes, the obtained phenol-aldehyde adhesives can show high weather resistance and boiling resistance, as well as a low formaldehyde release rate [
35]. In recent years, hydrolyzed tannins and condensed tannins have been widely explored in the field of adhesives. However, it is worth mentioning that the reactivity of hydrolyzed tannins with aldehydes is relatively low, and their connection units (such as ester bonds) possess relatively lower resistance to moisture [
38]. In contrast, condensed tannins show better resistance to moisture owing to their units linked by C-C bonds, which are widely used in the synthesis of adhesives and foams. In work carried out by Hafiz et al., the preparation of phenol-formaldehyde adhesives with different tannin (from Acacia mearnsii bark) concentrations was reported. The results showed that the introduction of tannin into the phenol-formaldehyde prepolymer accelerated the gelation time and increased the viscosity of the resins. Importantly, the dynamic thermomechanical analysis (DMA) results showed that the stiffness of all the target resins improved with the addition of tannin [
96]. In another study carried out by F. Santiago-Medina et al., the preparation of phenol-aldehyde adhesives for wood panels based on the reaction of procyanidin-type condensed tannins (from pine bark) and renewable vanillin-derived dialdehydes was reported. In this work, the target adhesive showed ideal gelation time, and it was suitable for fabricating particleboards (167 s at pH 10). This study provides a novel method to create totally non-toxic and environmentally friendly resins on the basis of renewable plant polyphenols and their derivatives [
97]. The reaction between condensed tannins and aldehydes can be catalyzed by an acid or base. Usually, the A ring (nucleophilic centers) is more reactive than the B ring, and the reaction of the B ring with aldehydes can be activated under alkaline conditions (approximately pH 10). It should be mentioned that the fast reaction of the A ring with aldehydes makes the subsequent process difficult to control (such as molecular weight, structure, viscosity, etc.); thus, the content of plant polyphenols in the system is limited to a low level for inhibiting the condensation reaction. Due to the incomplete polymerization, rigid and brittle resins (especially for phenol-aldehyde adhesives with unsatisfactory adhesion performance) may be obtained. To this end, the depolymerization of plant polyphenols before being used to synthesize phenol-aldehyde resins can effectively improve the performance of the target product. For instance, Li et al. reported a novel biobased adhesive (phenolic resin) with high performance, in which Acacia mangium tannin was depolymerized and then reacted with polyethyleneimine. The target adhesive exhibited shorter gelation time (67.6%) and fewer formaldehyde emissions (64.4%), as well as enhanced bonding strength (63.6%), compared with the control adhesive, owing to the ideal chemical reactivity of depolymerized tannin [
95]. Hopefully, the as-prepared biobased adhesive may be used as a substitute for traditional phenolic resins.
It is also possible to prepare phenol-aldehyde foams based on plant polyphenols. The traditional plant polyphenol-derived foam formulation mainly includes the following ingredients: plant polyphenol extract, formaldehyde, furfuryl alcohol (serving as an exothermic agent), blowing agent, water, additives, etc. In addition, glyoxal (a non-toxic di-aldehyde) can be regarded as a formaldehyde substituent to synthesize phenolic foams, avoiding the release of harmful gases. In work reported by Zhou et al., pine tannin was used as a biobased component, and glyoxal was used to completely replace formaldehyde for synthesizing eco-friendly pine tannin/furanic rigid foams, which exhibited low thermal conductivity and desired mechanical properties [
98]. As we know, depending on the formulation, plant polyphenol-derived foams can be deigned as rigid or semi-rigid types. It has been reported that the size and structure of the pores can be controlled by regulating the content of plant polyphenols (tannins) [
99].
To sum up, all kinds of works carried out to date about biobased phenol-aldehyde resins/foams composed of plant polyphenols are very promising, and the obtained products also show comparable thermal or/and mechanical properties to those current petroleum-based phenol-formaldehyde resins/foams.
3.4. Materials Based on Polyester
The preparation of plant polyphenol-derived polyesters is feasible via the reaction between plant polyphenols and different types of acylating agents such as carboxylic acids, acid anhydrides, and highly reactive derived monomers (e.g., acid halides) [
100]. The carbonization ability of plant polyphenols has been used in the preparation of polyesters with flame retardancy. In work carried out by Xia et al., the synthesis of flame-resistant bio-derived compounds from tannic acid was reported. As shown in
Figure 8a, tannic acid underwent a crosslinking reaction via interfacial polymerization with terephthaloyl chloride to obtain tannic acid-terephthalate, which exhibited good thermal stability (less than 3% mass loss at 230 °C), higher char yield of 36.4%, and low heat release ability (<80 J g
−1 K
−1). After coating tannic acid-terephthalate on nylon 66, the fabric showed rapid self-extinguishing capacity and a decreased char length in vertical combustion tests, due to the heat-resistant carbon layer created by tannic acid-terephthalate, which can effectively inhibit the flame propagation [
101]. This work confirms that tannic acid-terephthalate can be used as an ideal flame retardant to improve the fire resistance of fabric.
Plant polyphenols can also endow polyesters with good biodegradability. For example, P. Song and co-workers developed a novel biodegradable polyester on the basis of tannin-grafted polycaprolactone (TA-g-PCL). The results showed that the thermodynamic properties and dissolubility of TA-g-PCL polymer varied obviously with its molecular weight. With the growth of PCL monomer segments, the TA-g-PCL polymer was converted from the amorphous to crystalline state. At the same time, the dissolubility of the target TA-g-PCL polymer in chloroform was also significantly improved, which provided a possibility for its further application in the biomedical field [
102]. Altogether, this work presents a new perspective on the synthesis of novel biodegradable polyesters derived from plant polyphenols. Biobased polyesters can also be obtained through an interfacial polymerization reaction between gallic acid and five dicarboxylic dichlorides. The solubility and thermal properties of the prepared polyesters can be flexibly adjusted by selecting appropriate alkoxy groups and aromatic dicarboxylate units. Through special structure design, the glass transition temperature of the prepared polyesters can be controlled between 81 °C and 308 °C. In addition, as shown in
Figure 8b, the representative polyester exhibited the desired mechanical properties (elongation of 13.1% and Young’s modulus of 1.1 GPa) [
103]. In another study, Hakkarainen et al. developed sustainable polyesters based on a eugenol-modified 1,3-Dioxolan-4-one component. The target polyester showed the desired thermal stability (210 °C) and improved tensile strength (70.8 MPa), owing to the presence of the aromatic-rich structure in the system. In addition, the obtained polyester can be degraded in alkaline water. Importantly, benefiting from the transesterification between free hydroxyl groups and carboxyl groups, the prepared polyester showed ideal self-healing and shape memory abilities [
104].
Altogether, plant polyphenol-based polyesters can be simply fabricated by the reaction of plant polyphenols (and their derivatives) with different monomers (e.g., acid anhydrides, acid halides, fatty acids, etc.), which have been proven to show good flame retardancy, biodegradability, UV resistance, and so on. However, some functional polyesters obtained from plant polyphenols possessed relatively low flexural properties. Thus, for the next generation of plant polyphenol-derived polyesters, more attention should be paid to the balance of comprehensive performance.
Figure 8.
(
a) Schematic of the preparation of tannic acid-terephthalate. Reprinted from Reference [
101] with permission of Elsevier, Copyrights (2018). (
b) Stress–strain curve of the representative biobased polyester from gallic acid. Reprinted from Reference [
103] with permission of American Chemical Society, Copyrights (2019).
Figure 8.
(
a) Schematic of the preparation of tannic acid-terephthalate. Reprinted from Reference [
101] with permission of Elsevier, Copyrights (2018). (
b) Stress–strain curve of the representative biobased polyester from gallic acid. Reprinted from Reference [
103] with permission of American Chemical Society, Copyrights (2019).
3.5. Materials Based on Leather
As mentioned before, plant polyphenols can be regarded as vegetable tanning agents to achieve the conversion of hide into leather. In recent years, the interaction between plant polyphenols and collagens has been one of the research hotspots in the field of leather chemistry. In work reported by Teklemedhin et al., vegetable tannin was first extracted from Cassia singueana bark through a simple water extraction method (
Figure 9) and then further used for sheep pickle pelt. The obtained leather tanned by Cassia singueana tannin exhibited comparable physicochemical properties to traditional Mimosa tanned leather. For example, the shrinkage temperature, tensile strength, and elongation at break of the resulting leather reached up to 83 °C, 15.6 N/mm
2, and 45.3%, respectively, which were slightly superior to those of the control sample (tanned leather with Mimosa extract) [
105]. These results demonstrated that Cassia singueana extract can be used as an alternative eco-friendly tannin to alleviate the pollution caused by chrome tanning in the leather industry. Further, in work reported by Xiao et al., a sustainable metal-free combination tannage method based on triazine-based syntan bearing chlorine groups (SACC) and plant polyphenols (i.e., wattle, tara, quebracho, etc.) was developed (
Figure 10a). The obtained leather exhibited a high shrinkage temperature (~92 °C) that did not change obviously, even after being washed with water, urea, and n-propanol solution (
Figure 10b). It may be due to the fact that the SACC and plant polyphenol (wattle) can effectively diffuse into collagen fibers and bind to the collagen through covalent bonds, hydrogen bonds, and ionic bonds (
Figure 10c) [
106]. This work not only provides new ideas for improving the organoleptic and physicochemical properties of chrome-free leather products but also reduces the influences of chromium wastes to the environment and human health. In addition, in our previous work, a simple chrome-free combination tanning method by using silicic acid and plant polyphenols was proposed. The combination-tanned leather exhibited good thermal stability, desired mechanical properties and softness, as well as ideal storage stability compared to the control leather tanned by silicic acid alone. During the storage process, the Ph-OH groups of plant polyphenols can form a hydrogen bonding interaction with Si-OH groups to inhibit their excessive condensation. Importantly, the environmental impact assessment (including BOD
5/COD, TS, DS, and SS) of tanning wastewater demonstrated that this combination-tanning method is a green and sustainable tanning technique [
107]. With increasingly strict environmental regulations and policies, as well as the strengthening of people’s health awareness, the green tanning technology based on plant polyphenols will be further developed and applied in the leather field.
Plant polyphenols can not only be used for leather tanning but also used as functional components to endow leather products with the desired properties. In work reported by Marsal et al., the effect of plant polyphenols (mimosa, quebracho and tara) in the decrease of the formaldehyde concentration in leathers tanned by resin (melamine-formaldehyde or dicyandiamide-formaldehyde) was investigated. The results showed that mimosa possessed the desired capacity to decrease the formaldehyde concentration of the resin-tanned leathers, and the ability was enhanced with ageing [
108]. This may be due to the high reactivity of plant polyphenols towards formaldehyde. Furthermore, Yu et al. fabricated a novel high-performance water purification membrane based on tannic acid and collagen fibers derived from animal hides, in which tannic acid was used as a vegetable tanning agent to functionalize collagen fibrils inspired by the traditional leather tanning technique. The resulting composite showed ideal antibacterial properties because of the generation of reactive oxygen species (ROS) based on the catechol unit and possessed excellent water disinfection efficiency (>99.9%, ∼150 L m
−2 h
−1) [
109], which may provide new ideas for the preparation of green and antibacterial microfiltration membranes. In addition, Yan et al. reported a novel high-performance X-ray shielding material based on a microfiber membrane (a kind of synthetic leather with hierarchical structure) via the impregnation–desolvation strategy and coating process. In this work, with the assistance of plant polyphenols, the rare earth (Ce or Er) element can be effectively loaded and uniformly dispersed into the microfiber membrane. The obtained composites showed an average 10% X-ray attenuation efficiency higher than the control sample excluding polyphenols, and an improvement of 9% in X-ray attenuation efficiency compared to that without hierarchical structure. Due to the synergistic effect between plant polyphenols and the hierarchical structure, the target composite bearing uniformly dispersed rare earth element showed an average improvement of 19% in X-ray attenuation efficiency compared to that of lead sheet [
110]. Inspired by this work, leather/collagen-based products with X-ray shielding capability have the potential for further application in the clinical field.
To sum up, as a rich and renewable resource, plant polyphenols can be used as vegetable tanning agents to reduce the contamination caused by chrome tanning agents and can also be worked as functional components to endow leather with satisfactory properties, showing great potential for application in the leather industry.
3.6. Materials Based on Hydrogel
As a kind of soft material, hydrogels composed of a crosslinked network and hydrophilic component can absorb and retain an adequate amount of water [
111,
112,
113]. Due to the unique properties (such as good biocompatibility, ideal water absorption, and tunable mechanical properties), hydrogels have been widely applied in various fields, including medicine, tissue engineering, sensors, and actuators [
114,
115,
116,
117,
118,
119]. In recent years, there have been more and more reports on the preparation of hydrogels from plant polyphenols. Benefiting from their excellent properties, the obtained plant polyphenol-derived hydrogels will also show various functions.
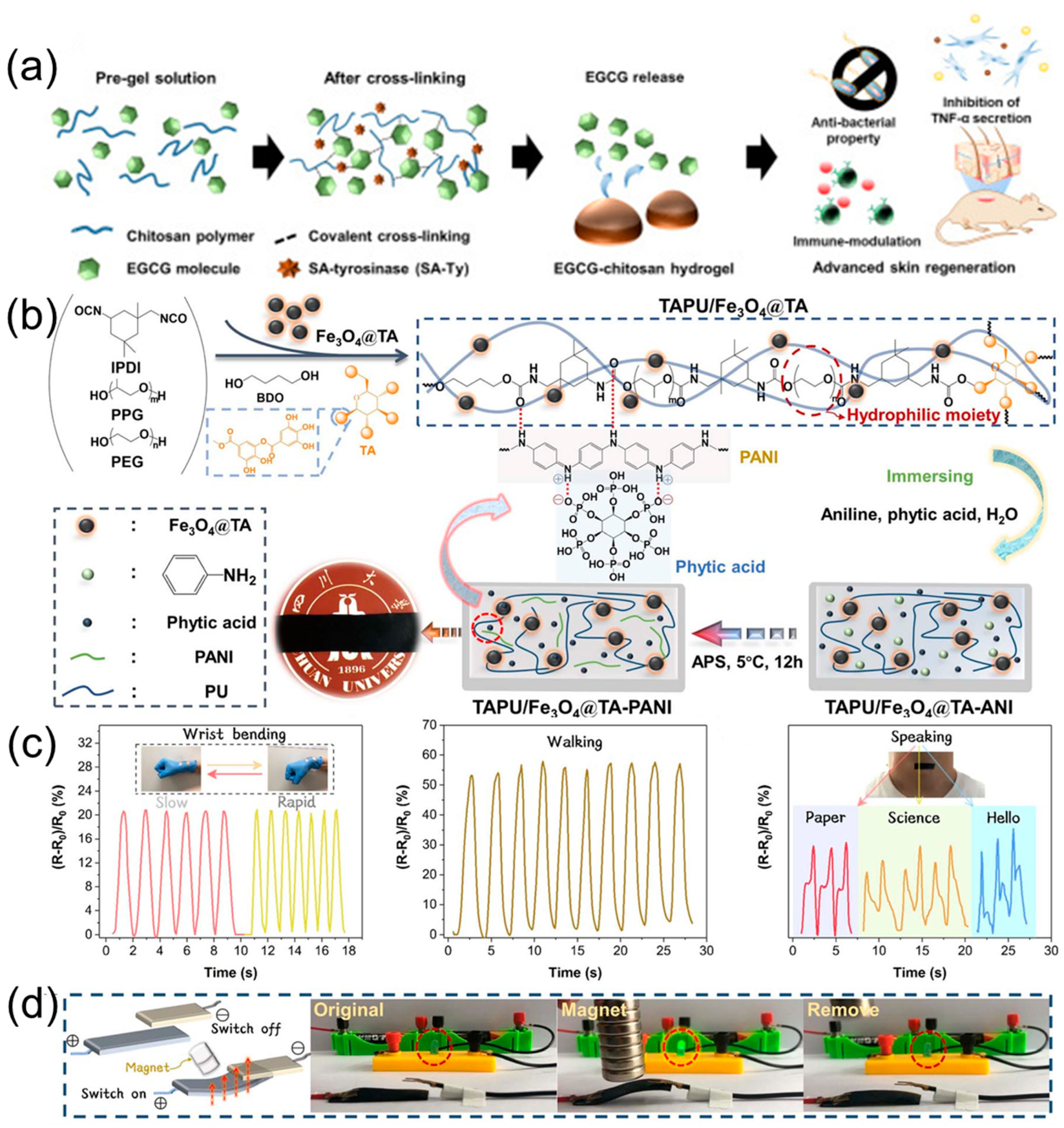
For instance, in work reported by Zhang et al., a robust and fully green hydrogel with a water content of 54% was successfully prepared based on chitosan and tea polyphenols, which showed good antibacterial and antioxidant properties as well as UV resistance and can be applied to soft contact lens [
120]. Fully green polyphenol-derived hydrogels may show desired biocompatibility and have important research value in the biomedical field. Furthermore, plant polyphenols can induce immunity in humans and inhibit external infections. Kim et al. developed a novel biobased hydrogel containing epigallocatechin gallate (EGCG) and chitosan through a one-pot method (
Figure 11a); its crosslinked degree and physical properties can be adjusted by the EGCG concentrations. Importantly, the obtained hydrogel showed desired antibacterial and antioxidant properties, which can be utilized in full-skin defect wounds to promote skin regeneration due to the EGCG having the ability to scavenge free radicals and suppress inflammation [
121]. Moreover, in work carried out by Deng et al., an agarose-based nanocomposite hydrogel with tannic acid-Fe
3+ component was developed, which showed excellent mechanical properties and biocompatibility, as well as an ideal photothermal effect (58 °C, irradiating for 10 min under NIR light). Interestingly, the prepared hydrogel can kill ~99% of bacteria after being irradiated by NIR light for 10 min and effectively promote wound healing [
122], showing great potential as a functional wound dressing in the biomedical field. The above studies demonstrated that the introduction of plant polyphenols can regulate the intrinsic properties of the target hydrogels, and the further construction of a coordinative network based on plant polyphenols and metal ions will also endow hydrogels with special functionality.
Recently, some other multifunctional hydrogels (such as self-adhesives, sensors, and actuators) have also been designed based on plant polyphenols. For example, Fan et al. prepared a self-adhesive, antibacterial, and recyclable dual-network hydrogel based on PVA/chitosan/cyclodextrin/black wattle tannin (a kind of low-cost plant polyphenol), which exhibited ideal mechanical properties and fatigue resistance, as well as high sensitivity (GF = 4.87). Importantly, the incorporation of black wattle tannin into the hydrogel matrix can endow it with excellent and repeated adhesion. The resulting hydrogel can be worked as a flexible strain sensor to detect human movements [
123]. Similarly, in work reported by Liu et al., a dual-network interpenetrating conductive hydrogel based on poly(acrylic acid), methacrylic acid-functionalized chitosan, Fe
3+ ions, and tara tannin was successfully synthesized, which possessed high transmittance, ideal UV absorption capacity, good mechanical properties (900%, 0.75 MPa), and desired adhesion. In addition, its excellent self-healing ability and strain sensitivity (GF = 5.33) made it possible to serve as a conductive strain sensor [
124]. Zhang et al. fabricated an NIR-responsive composite hydrogel by introducing Fe
3+/tannic acid (photothermal agent) into the poly(N-isopropylacrylamide) matrix. The obtained hydrogel exhibited good mechanical properties and photothermal effects due to the formation of a metal–phenolic network based on Fe
3+ ions and tannic acid. Benefiting from the desired thermosensitivity (the volume phase transition temperature is about 32 °C) and photothermal conversion ability, the resulting hydrogel can achieve the deformation behaviors under NIR irradiation and can be used as NIR-responsive actuators [
125]. Combining the advantages of plant polyphenols with other functional components will undoubtedly further expand the application area of polyphenol-based hydrogels. In our previous work, a magnetic-responsive, environmentally stable, and conductive organohydrogel was successfully prepared. Firstly, Fe
3O
4 nanoparticles were modified by tannic acid and then further introduced into a tannic acid-based polyurethane matrix bearing PEG units (hydrophilic component) through in situ polymerization. Subsequently, the as-prepared nanocomposite film was soaked in an aqueous solution containing aniline and phytic acid to obtain the nanocomposite hydrogel with improved conductivity (
Figure 11b). Finally, the target nanocomposite hydrogel was converted into organohydrogel through a solvent exchange strategy, which showed ideal conductivity (0.36 mS cm
−1), mechanical properties (371%, 0.83 MPa), environmental tolerance, and electromagnetic shielding effectiveness. Interestingly, the resulting organohydrogel can be worked as a strain sensor to monitor human movements (
Figure 11c) and designed as a magnetically controlled switch to achieve circuit connection (
Figure 11d) [
126].
To sum up, plant polyphenols can be broadly used for preparing multifunctional hydrogels (e.g., as wound dressing, strain sensors, and stimuli-responsive actuators) due to their unique features, including biocompatibility, high reactivity, adhesion, chelating ability, and so on. We believe that with the deepening of research on plant polyphenol–material system, more and more intelligent hydrogels will appear and show great application potential in various fields.
Figure 11.
(
a) Schematic of the preparation of EGCG-chitosan hydrogel. Reprinted from Reference [
121] with permission of Elsevier, Copyrights (2020). (
b) Schematic of the preparation of nanocomposite hydrogel. (
c) Relative resistance changes of the target organohydrogel for monitoring human movements. (
d) Photography of an electrical switch triggered by using a magnet. Reprinted from Reference [
126] with permission of Royal Society of Chemistry, Copyrights (2023).
Figure 11.
(
a) Schematic of the preparation of EGCG-chitosan hydrogel. Reprinted from Reference [
121] with permission of Elsevier, Copyrights (2020). (
b) Schematic of the preparation of nanocomposite hydrogel. (
c) Relative resistance changes of the target organohydrogel for monitoring human movements. (
d) Photography of an electrical switch triggered by using a magnet. Reprinted from Reference [
126] with permission of Royal Society of Chemistry, Copyrights (2023).
3.7. Materials Based on Nanoparticles
Nowadays, organic/inorganic/organic–inorganic nanoparticles are widely applied in the catalysis, electronics, environment, medicine, and composite material fields. Plant polyphenols can also be used to prepare nanoparticles based on their self-polymerization or complexation reaction with metal ions. In work reported by Zhao et al., novel poly(tannic acid) nanoparticles with intrinsic fluorescence were successfully prepared based on the self-polymerization of tannic acid, which exhibited good water dispersibility, ideal biocompatibility, and biodegradability. Importantly, the fluorescence of the obtained poly(tannic acid) nanoparticles can be quenched using picric acid, demonstrating a sensitive and rapid detection ability (as a fluorescent sensor) [
127]. In addition, Yang and co-workers fabricated a series of natural polyphenol-based fluorescent polymer dots through a one-pot co-polymerization strategy without an external energy input, which showed ideal photostability and biocompatibility. The luminescence performances of the target polymer dots varied with the structure of polyphenols. Importantly, the resulting polymer dots possessed high selectivity towards Cu
2+, which can be applied in the detection of Cu
2+ (as fluorescent probes) in living organisms and environmental water samples [
128]. This work provides a new idea and sustainable avenue for the creation of biomass fluorescent polymer dots derived from plant polyphenols, which shows great potential in the biomedical and environmental applications (especially for pollutant detection). In another work, a novel template-mediated supramolecular assembly strategy was proposed to prepare protein-polyphenol-based nanoparticles [
129]. After removing the mesoporous template, the obtained biomass nanoparticles can achieve charge reversal (in acidic condition) and glutathione-induced disassembly via competitive supramolecular interactions, which can be used for intracellular protein delivery. In the field of biomedicine, the intracellular delivery of proteins has been regarded as a promising strategy to control cellular behavior. Currently, although some intracellular delivery methods have been developed, designing a polyphenol-based versatile delivery system that can respond to different physiological triggers through simple chemical synthesis should be given more attention. In another interesting work reported by Wang et al., a facile one-step strategy was proposed to achieve the “hydrophobic-to-superhydrophilic” change of commercial films through the co-deposition of 3-aminopropyltriethoxysilane (APTES) and tannic acid. Benefiting from the good adhesion of tannic acid, the hydrophilic and hierarchical nanospheres formed by self-assembly (based on Michael addition reaction and Schiff’s base reaction) can be loaded onto the hydrophobic films (such as PVDF, PTFE, and PP). Moreover, the obtained superhydrophilic commercial films achieved the high-efficiency separation of oil-in-water emulsions (
Figure 12a) [
130]. The above functional nanoparticles were obtained based on the chemical reaction of plant polyphenols with organic monomers. Impressively, metal ions can also participate in the construction of plant polyphenol-derived nanoparticles.
In work reported by Liu et al., novel biodegradable EGCG-Fe
3+/PVP (EFPP) nanoparticles possessing ultra-small size and ideal biocompatibility were prepared based on the complexation effect of EGCG, Fe
3+ ions, and PVP (
Figure 12b), which showed good suppression of Aβ40 fibrillation and can also decompose existing Aβ40 fibrils owing to the weak hydrophobicity of PVP and antioxidant activity from EGCG [
131]. The obtained EFPP nanoparticles had great application potential in the treatment of Alzheimer’s disease. Similarly, Zeng et al. fabricated novel pH-sensitive Fe
3+-gallic acid nanoparticles, which exhibited strong NIR absorbance. The size of the nanoparticles can be controlled by adjusting the solution pH value. The obtained nanoparticles were unstable under neutral conditions but stable under mild acidic conditions (pH ~5.0), which can be used for cancer diagnosis and treatment. In addition, due to the excellent photothermal effect and low toxicity, Fe
3+-gallic acid nanoparticles had the potential as a biomedical photothermal agent [
132]. In addition, Wang et al. synthesized a series of Fe
3+-phenol nanoparticles based on plant polyphenols having different structures through a facile one-step complex method. The results showed that the prepared Fe
3+-pyrocatechol and Fe
3+-tannic acid nanoparticles possessed the desired photothermal conversion capacity and can further lead to MCF-7 cell death under laser irradiation, as well as exhibiting enormous potential as photoacoustic agents [
133]. To date, various polyphenol-based nanoparticles can be designed and prepared via intra- and intermolecular interactions of plant polyphenols. In addition, the morphology and size of polyphenol-based nanoparticles with or without other macromolecules/metal ions can also be regulated by these diverse and reversible interactions, including self-assembly, polymerization, and coordination. Benefiting from their good biocompatibility, polyphenol-based nanoparticles have shown great advantages in medical fields, such as drug delivery, magnetic resonance imaging, photoacoustic imaging, and so on.
As mentioned before, the abundant catechol groups in plant polyphenols can chelate with different types of metal ions to achieve the construction of multifunctional nanoparticles. Importantly, the primary nanoparticles can be first prepared based on small-molecular plant polyphenols through a variety of intermolecular interactions (including host–guest interaction, hydrophobic interaction, self-polymerization, etc.), which are rich in Ph-OH groups. Therefore, the secondary structured nanoparticles can be further constructed through metal ion modification on their basis. Altogether, the multi-level polymerization/self-assembly strategy is of great significance for the in-depth understanding of the assembly process from the molecular to nano (or macro) scales and also provides a reliable method for the construction of advanced smart materials.
3.8. Other Functional Materials
In addition to the above methods for preparing plant polyphenol-based materials, other pathways of obtaining multifunctional biobased materials with fascinating applications in the food, environmental, and biomedical fields have also been reported [
134,
135,
136,
137].
For instance, in the work published by Yuan et al., an active edible film was developed by introducing tea polyphenols into calcium alginate gel. The results showed that with an increase in the tea polyphenol amounts, the mechanical properties and water vapor permeability were improved. Additionally, after introducing tea polyphenols, the target film exhibited enhanced antioxidant capacity and anti-inflammatory properties, which had great potential in the food packaging field [
138]. In addition, Yin and co-workers fabricated an active and intelligent collagen-based packaging film with collagen, delphinidin (polyphenolic compound), and laccase, which showed good dry/wet tensile strengths (41.74 MPa and 13.13 MPa), antioxidant ability, and barrier properties. Benefiting from the good antioxidant activity of the polyphenolic compound, the packaging film can extend the shelf life of food. Importantly, after further incorporating vaccinium oxycoccus pigment (another type of polyphenolic compound), the target packaging film can be used to monitor the freshness of protein/fat-rich food through color changes, owing to the different structures of vaccinium oxycoccus pigments at different pH conditions (pH-sensing indicator) [
139]. Consequently, based on the antioxidant capacity and pH responsiveness of polyphenolic compounds, the plant polyphenol-derived functional films will show great application potential in the field of smart food packaging. Furthermore, regarding the environment, Deng et al. reported a new nano-sized adsorbent for Pb
2+ based on the polyphenolic condensation of tea polyphenols, which showed a relatively high removal efficiency (>90%, 584.8 mg/g at pH 5.5 and 25 °C) due to the presence of abundant Ph-OH groups [
140]. The method in this work demonstrated that the tea polyphenols can be further applied in the removal of heavy metal ions from wastewater.
In terms of plant polyphenol-derived functional polymers, Bai et al. prepared a new type of triple-responsive shape memory polymer based on the crosslinked poly(vinyl alcohol) and tannic acid/Fe
3+ complex (
Figure 13a). In this work, benefiting from the hydrophilic poly(vinyl alcohol) and excellent photothermal conversion capability of the tannic acid/Fe
3+ complex, the resultant polymer can achieve good water-, thermal-, and NIR light-triggered shape memory properties, where the shape recovery ratios triggered by the above three stimuli reached over 95% [
141]. Plant polyphenols can also be used for fabric finishing. For example, Jiang et al. fabricated a new phosphorus–nitrogen flame retardant on the basis of tea polyphenol, melamine, and phenylphosphonic acid (
Figure 13b), which can generate free radicals and non-flammable gases (such as N
2 and NH
3) during combustion. The results showed that the cotton fabrics coated with the flame retardant possessed good flame retardancy (the LOI value reached to 28.7%, the peak heat release rate was reduced by 88.5%) and UV resistance [
142]. The method in this study provided a good alternative for the preparation of a green flame retardant. In addition, plant polyphenols can be further applied in the field of biomedicine. In work reported by Yu et al., a low-cost biobased cryogel was successfully prepared by using tannic acid/Fe
3+ as the photothermal agent and chitosan-silk fibroin as the skeleton (
Figure 13c). Due to the effect of the hydrogen bond and coordination bond, the prepared cryogel showed ideal flexibility and recoverability. Meanwhile, benefiting from the porous structure of the matrix and photothermal conversion ability of the tannic acid/Fe
3+ complex, the target cryogel can be used as a wound dressing to absorb blood for hemostasis and kill bacteria, as well as promoting skin regeneration [
143]. It can be reasonably inferred that plant polyphenol-derived wound dressing will demonstrate greater potential for clinical applications due to their excellent comprehensive performance. In addition, Kohli et al. developed a dental bleaching agent with polyphenols (strawberry extract), which showed ideal bleaching properties and antimicrobial efficacy [
144]. Due to the acidic properties of polyphenolic compounds, they can serve as strong oxidizing agents on the surface of tooth enamel.
There are many other applications of plant polyphenols, such as in the fields of drug delivery [
145,
146,
147], nanocoating [
148,
149,
150], cancer treatment [
151,
152,
153], etc. The latest works will be further systematically summarized and discussed in future reviews. In a word, due to the high chemical reactivity and unique properties of biobased plant polyphenols, they have been widely utilized in various multifunctional polymeric materials.