1. Introduction
Probiotic application is widespread in humans and animals for their anti-inflammatory and immunomodulatory response, as well as improved gut health [
1,
2,
3]. The definition of probiotic in the root sense means “for life”, and the critical expectation is that live and active cultures should be delivered in substantial amounts to observe health benefits [
4,
5]. Some probiotic bacteria are predominant residents of the gut [
6]. Probiotics have desirable attributes that benefit the host, such as producing short-chain fatty acids (SCFAs), immunomodulation, surviving acidic environments, pathogen exclusion, and influencing the microbiome composition [
7,
8]. Probiotic bacteria have been used in human food or animal diets to prevent pathogen colonization and improve gut health through competitive exclusion [
9,
10,
11]. Probiotics are now used as an additive to kibble and wet foods for companion animal gut health [
2,
12]. Their beneficial effects are either transient or ineffective [
2,
13], primarily due to their inability to colonize the inflamed bowel [
14,
15,
16]. Probiotics have also been used to sequester mycotoxin from feed [
17,
18,
19]. Therefore, precedence exists for exploring further characterization of probiotic additives in companion animal feed.
Canines are exposed to numerous pathogenic and environmental insults that make them vulnerable to illness. Dog food has been linked to multiple outbreaks associated with
Salmonella enterica,
Listeria monocytogenes,
Escherichia coli, and other pathogens [
20,
21,
22]. In the dysbiotic gut, pockets of inflammation and dysregulated epithelial cell tight junction permeability are evident [
23]. The inflamed state of the bowel propels the reconstruction of the enteric flora to that of a negative state, termed dysbiosis [
24]. Canine gut dysbiosis has shown an imbalance in the standard phyla of bacteria, including
Firmicutes,
Fusobacteria,
Bacteroidetes,
Proteobacteria, and
Actinobacteria [
25,
26].
Using probiotic bacteria with their beneficial properties as a canine food additive is one of the attractive methods of reducing pathogen exposure, as well as antibiotic use and promoting gut health [
2,
16]. Clinical trials for probiotics in pet food have been carried out, and several products on the market contain stable probiotics that may promote pathogen clearance and improve gut health while promoting overall animal fitness [
27].
Natural probiotics may have limited capabilities of colonizing the inflamed-damaged tissue; therefore, recombinant bioengineering has been used as a novel strategy to enhance probiotic epithelial adhesion, biofilm formation, immunomodulation, and pathogen colonization resistance [
14,
28,
29]. Nevertheless, genetically engineered and native probiotics possess the same inherent properties for pathogen exclusion, such as nutrient partitioning, biofilm formation, and clusters of abundance within the gastrointestinal (GI) tract [
30].
Biofilm formation by pathogenic or beneficial bacteria aids in their adhesion and colonization to biotic and abiotic surfaces [
31,
32,
33]. Since probiotics are natural gut inhabitants, they can form a biofilm on the mucosal surface, often in mixed communities, which is a vital and desirable attribute [
32]. During biofilm formation, bacteria communicate via the messaging system termed quorum sensing [
32,
34,
35]. Probiotic biofilms promote gastrointestinal healing, community structure, and the immunomodulatory response [
36]. Bacteria produce exopolysaccharides, proteins, and extracellular DNA to aid in biofilm formation [
33]. Biofilms produced by probiotics can competitively inhibit pathogen colonization, such as seen with the
Listeria monocytogenes challenge in a mouse model [
14]. Furthermore, biosurfactants from
Lactobacillus jensenii and
Lacticaseibacillus rhamnosus have shown antimicrobial and antibiofilm formation properties against clinically relevant strains of
Acinetobacter baumannii,
Escherichia coli, and
Staphylococcus aureus (MRSA) [
37]. A cocktail of
Lactobacillus fermentum and
Lactiplantibacillus plantarum could also reduce
S. aureus (MRSA) biofilm production [
38].
Therefore, in this study, we aimed to evaluate antimicrobial activities, epithelial cell adhesion, biofilm formation, and the anti-inflammatory response of a canine probiotic blend (LabMAX-3) that contains
Lactobacillus acidophilus,
Lacticaseibacillus casei, and
Enterococcus faecium, which are known to promote canine gut health [
39,
40,
41], using a Madin-Darby Canine Kidney (MDCK) cell line. MDCK cells are derived from the renal tubule of the canine kidney and display enterocyte-like absorbing properties; thus, they are widely used as a canine gut epithelial cell model [
42,
43].
2. Materials and Methods
2.1. Probiotic Preparation
A commercial proprietary probiotic blend, LabMAX-3, containing equal proportions of three probiotic cultures of
Enterococcus faecium,
Lactobacillus acidophilus, and
Lacticaseibacillus casei, was received from CH2 Animal Solutions (Ottumwa, IA, USA). Since the lyophilized powder blend (LabMAX-3) could not be directly used in our proposed experiments due to the presence of some insoluble materials (part of formulations), we isolated each culture from the blend and mixed them at a 1:1:1 ratio, designated LabMAX-3. The lyophilized powder was plated on DeMann Rogosa Sharpe (MRS, Thermo Fisher Scientific, Walthan, MA, USA) agar plates and incubated at 37 °C for 48 h under anaerobic conditions using a Gaspak (Thermo Fisher Scientific). The colonies were picked based on phenotype, and each bacterial culture morphology was verified by microscopy (Leica, Deerfield, IL, USA).
L. acidophilus NRRL 31910,
Pediococcus acidilactici H, and
E. faecium ATCC 8459, as controls, were propagated in MRS under anaerobic conditions.
Lacticaseibacillus casei ATCC 334 was cultivated in modified MRS agar (MMRS) containing 1%
w/
v proteose peptone, 0.5%
w/
v yeast extract, 0.1%
v/
v Tween 80, 0.2%
w/
v meat extract, 37 mM C
2H
3NaO
2, 8.8 mM C
6H
14N
2O
7 dissolved in 0.2 M potassium phosphate (dibasic, pH 7.0), 0.8 mM MgSO
4, 0.24 mM MnSO
4, and 1%
w/
v mannitol and supplemented with erythromycin (2 µg/mL, Thermo Fisher Scientific) and grown in anaerobic conditions [
14].
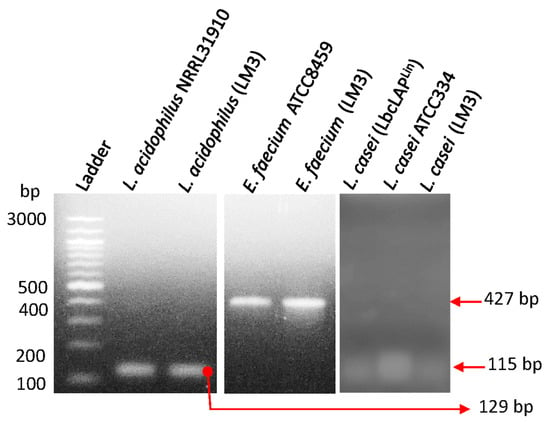
2.2. PCR Confirmation of Probiotic Strains
Probiotic culture isolates in LabMAX-3 were confirmed by PCR amplification of the species-specific target genes listed in
Table 1. Target gene amplification was performed using PCR Master Mix (Platinum™ Hot Start, Thermo Fisher Cat# 130000). An equal volume of template (2 µL of resuspended colony) was added, and DNA was amplified via a thermocycler (Applied Biosystems, Carlsbad, CA, USA). Thermocycling conditions are as follows: 94 °C for 10 min, 30 cycles at 94 °C at 30 s per cycle, 30 s at 60 °C, 72 °C at 30 s, and 5 min at 72 °C. PCR products were analyzed by agarose gel electrophoresis (2%), and DNA bands were visualized under UV exposure using a Kodak EDAS 290 camera (Rochester, NY, USA) with ID LE 3.6.3 software.
2.3. Pathogen Propagation
Cultures of enterotoxigenic
Escherichia. coli (ETEC) strains F4 and O78:H11,
Staphylococcus aureus ATCC 25923
Salmonella enterica serovar Typhimurium ST1, and
Listeria monocytogenes F4244 from our culture collection (
Table 2) were cultivated in Tryptic Soy Broth supplemented with 0.6% yeast extract (TSBYE) at 37 °C for 18–24 h.
Campylobacter jejuni ATCC 29428 was cultivated in microaerophilic conditions (5% CO
2, 37 °C, 7 days) in Bolton Broth (Neogen, Lansing, MI, USA).
2.4. Antimicrobial Activity Testing on Agar Plates
The antimicrobial activity of individual probiotic cultures of
Enterococcus faecium,
Lactobacillus acidophilus, and
Lacticaseibacillus casei in a mixture (1:1:1) of the three designates LabMAX-3,
P. acidilactici H, and
L. casei ATCC334 was tested against pathogens (
Table 2) as before [
46]. Briefly, overnight-grown (37 °C for 18 h) cultures of probiotic strains were inoculated (1 µL, stab method) on a base layer of MRS agar and incubated for 18–20 h at 37 °C anaerobically. Pathogens were grown at 37 °C for 24 h in TSBYE aerobically. Tryptic Soy Agar supplemented with 0.6% yeast extract (TSAYE, Thermo Fisher Scientific) soft top agar (0.8% agar) was prepared, and 10 µL of the respective pathogens were inoculated and vortexed. The inoculated soft agar (5 mL) was then poured onto the base layer, swirled, air-dried, and incubated at 37 °C for 24 h. The zones of inhibition around the stab culture were measured.
For the preparation of cell-free supernatants, probiotic bacterial culture (18–20 h grown as above) supernatants were collected after centrifugation (14,000×
g, 5 min) and passed through 0.45 µm membrane filters. A 20 µL aliquot was tested on a sterile blotting paper disc against the test pathogens that were overlaid on TSA agar plates [
46].
In separate experiments, each 18–20 h-grown probiotic culture was heat-treated (80 °C for 10 min), and 10 µL of each culture was placed on the BHI agar surface and overlaid with the test pathogens as above [
46].
2.5. Biofilm Formation by Probiotics
Biofilm formation by probiotic blend LabMAX-3 was analyzed in multi-well polystyrene plates as before [
47]. Briefly, anaerobically grown probiotic bacteria were suspended in a 1:1 ratio of MRS and MMRS, dispensed into 96-well tissue culture plates at 1.0 × 10
8 CFU/well (TPP, Trasadingen, Switzerland), and incubated at 37 °C for 24, 48, and 72 h anaerobically.
Lacticaseibacillus casei ATCC 334 and
Staphylococcus aureus were used as positive controls and grown in MRS and TSBYE, respectively, and inoculated at 1 × 10
8 CFU/well in a 1:1 ratio of MRS:MMRS or MRS:TSB, respectively, then incubated at 37 °C for 24, 48, and 72 h aerobically. Every 24 h, the old media were aseptically replaced with fresh media. Biofilms were washed three times in 0.2 mM phosphate-buffered saline, pH 7.2 (PBS), to remove planktonic bacteria. Bacterial counts in the biofilm were enumerated after scraping the biofilms from the well; collecting the cells in an Eppendorf tube; sonication for 20 min using iSonic Model #P4830 set at Frequency 60 Hz, Watt 150, Volt 110–120, and waveform 18.3 (Chicago, IL, USA); and serially diluted for plating on MRS or TSAYE agar plates [
31].
The formation of biofilms was also assayed by crystal violet staining. Briefly, washed biofilms were stained with 1% crystal violet (Sigma, St Louis, MO, USA), washed three times in PBS, air-dried for 15 min at room temperature (~25 °C, RT), treated with 95% ethanol, and the absorbance (OD590
nm) of supernatant was measured [
47].
In a separate experiment, bacterial biofilms were allowed to form in multiwall chambered glass slides (LabTek II, Cat# 154534, Thermo Fisher) that were UV-pretreated for 45 min. Wells were washed 3× in PBS, air-dried for 15 min at RT, heat-fixed, subjected to Gram staining using crystal violet without the counter staining, and visualized under a Leica Microscope (Deerfield, IL, USA) with 1000× magnification.
2.6. MDCK Cell Line Preparation
The Madin-Darby Canine Kidney (MDCK, NBL-2, CCL-34, ATCC, Manasas, VA, USA) cell line was cultivated in Dulbecco’s Modified Eagles Medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% Fetal Bovine Serum (FBS, Gibco), termed D10F. The canine cell line was grown at 37 °C at 5% CO2, passaged from 4–15, and propagated in T-75 flasks (TPP, Switzerland) until 75% confluence was reached. The cells were treated with Trypsin-EDTA (0.25%) (Gibco), centrifuged (800× g for 2.5 min), and resuspended in D10F. The MDCK cells were counted by 0.4% Trypan blue (Gibco) staining and seeded at a density of 104/well.
2.7. Treatment of MDCK with Lipopolysaccharide
Lipopolysaccharide (LPS, 1 mg/mL, Sigma, St Louis, MO, USA) was reconstituted in sterile deionized (d) H
2O and aliquoted for one-time usage. MDCK cells were treated with LPS at 1 µg/mL in D10F for 24 h at 37 °C and 5% CO
2 to induce modest inflammation [
43]. The monolayers were washed three times in DMEM to remove the LPS present and then treated with probiotics. Control wells (no LPS) received equivalent amounts of dH
2O to serve as a vehicle control.
2.8. Probiotic Adhesion to MDCK Cell Line by Plate Counting
MDCK cells were seeded at 1 × 104 cells/mL/well and cultured for 9 days to allow monolayer formation. Cell monolayers were washed three times in DMEM and treated without (no LPS groups) or with freshly prepared D10F containing 1 µg/mL LPS and incubated for 24 h (37 °C, 5% CO2). Monolayers were then washed three times in DMEM to remove residual LPS, and cells were examined for monolayer integrity by microscope.
Probiotics were grown anaerobically for 24 h in MRS or MMRS as before, added to MDCK cell monolayers with a multiplicity of exposure (MOE) of 1000, and incubated at 37 °C with 5% CO2 under humidified conditions for 24 h. Supernatants were collected for lactate dehydrogenase (LDH) and cytokine profiles (see below). MDCK cell monolayers were washed three times in DMEM to remove unattached bacteria. Cell monolayers were detached using Triton-X 100 (Sigma) treatment (0.1% for 5 min), vortexed, serially diluted, plated, and incubated at 37 °C for 48 h anaerobically. Colony counts were plotted to determine bacterial adhesion.
2.9. Adhesion and Biofilm Analysis by Giemsa Staining
MDCK cells were seeded (1× 104 cells/mL/chambered well) in UV-pretreated (45 min) cassettes (LabTek II, Cat# 154534, Thermo Fisher), incubated at 37 °C at 5% CO2 for 9 days, and inoculated with bacteria at MOI 1000, as above. The cell monolayers were fixed with 100% methanol for 10 min, air-dried, and stained with Giemsa stain (1:20 dilution in dH2O and methanol, stained for 45 min, air-dried, and visualized under the Leica microscope, Leica, Wetzlar, Germany).
2.10. Lactate Dehydrogenase Assay
Lactate dehydrogenase (LDH) release was from MDCK cells, and percent cytotoxicity was calculated as per the manufacturer’s instructions (Cayman Chemicals, Ann Arbor, MI, USA) [
14].
2.11. Cytokine ELISA
Canine cytokines IL-10, TGFβ, and TNFα were purchased from R&D Systems (Minneapolis, MN, USA), and cytokine levels in MDCK culture supernatants were quantified following the manufacturer’s instruction.
2.12. Data Analysis
Data were analyzed with GraphPad Prism (La Jolla, CA, USA) with unpaired Mann–Whitney tests. All data sets are representative of at least three experimental/biological replicates. Data are presented with standard error of the mean (±) or box and whisker plots with an interquartile range.