1. Introduction
Mammary neoplasms are the most common group of tumors encountered in veterinary practice, exhibiting a biological behavior that ranges from benign to highly malignant. Metastases are reported in 25 to 50% of cases, emphasizing the aggressive potential of these tumors. Mammary cancer in dogs serves as an exceptional model for studying breast cancer in women due to striking similarities in tumor histology, disease progression, behavior, associated risk factors, and hormone receptor profiles [
1].
These neoplasms often originate as benign lesions. However, under certain conditions, they may accumulate genetic and epigenetic changes that drive their progression to malignancy. On the other hand, malignant mammary tumors typically exhibit an aggressive phenotype from the moment of diagnosis, including invasiveness and metastatic potential. Studies report that approximately 25% of dogs experience the development of new nodules following surgical removal of initial tumors. Interestingly, these subsequent nodules may display malignant characteristics, even when the primary lesion was histologically classified as benign [
1]. As previously reported [
2], hormonal stimulation—often associated with recurrent pseudocyesis—can exacerbate this process. In cases where tumors are not surgically removed promptly, hormonal influence may lead to tumor disorganization, the accumulation of alterations and mutations, and ultimately, a worse prognosis. Delays in surgical excision increase the likelihood that these tumors will undergo malignant transformation, further complicating treatment and prognosis [
3].
Mammary neoplasms mainly affect middle-aged and elderly female dogs that have not been spayed. Breeds such as Poodles, Dachshunds, Yorkshire Terriers, Cocker Spaniels, German Shepherds, Boxers, Fox Terriers, and mixed-breed dogs are predisposed to developing these tumors. Malignant mammary neoplasms, particularly carcinomas, are characterized by an aggressive inflammatory process, rapid growth, regional lymphatic infiltration, and metastasis to distant tissues [
3]. The lymphatic system is the primary route of dissemination, with the lungs being the most commonly affected site in metastatic cases. Consequently, thorough evaluation using complementary diagnostic exams is essential for accurate staging and treatment planning [
4].
Cytological criteria, such as variation in nuclear size, prominent nuclei, altered nuclear-to-cytoplasmic ratios, and variations in the number, shape, and size of the nucleoli are important predictive factors of malignancy. However, one of the main challenges in obtaining an accurate cytological diagnosis in mammary neoplasms in dogs is the significant heterogeneity of tumor tissue. This heterogeneity arises from the presence of necrotic and inflamed regions, which can distort cellular morphology, as well as from distinct niches of tumor cells displaying varying levels of genetic and phenotypic alterations. Such variability complicates the interpretation of cytological features and may obscure the distinction between benign and malignant characteristics [
5].
Dogs have five pairs of mammary glands, and the incidence of multiple mammary tumors has been reported to reach as high as 60%. Despite the relatively high occurrence of synchronous mammary tumors, the relationship between these tumors remains poorly understood. Most reports suggest that they are independent primary tumors with distinct histopathological features, leading to the recommendation that all tumors be biopsied to assess malignancy. Therefore, benign and malignant tumors are generally considered separate entities, with the former not acting as precursors to the latter. However, recent studies challenge this assumption. One publication reported an increased risk of developing new malignant primary tumors in other mammary glands in dogs with a previous diagnosis of mammary carcinoma compared to those with benign tumors. Additionally, another study reported a high incidence of concurrent carcinoma in situ in dogs diagnosed with carcinomas [
6].
The search for biomarkers in canine mammary tumors is particularly relevant for predicting tumor progression in both species, providing insights into shared mechanisms of carcinogenesis between dogs and humans. These similarities emphasize the importance of identifying early diagnostic biomarkers for metastasis and prognostic markers that can guide clinical practice, ultimately improving patient outcomes in both species.
4. Materials and Methods
4.1. Samples and Histological Processing
The tissue blocks selected for this study were previously submitted to histopathological evaluation (hematoxylin–eosin stain) by the clinic from which they were acquired, focusing on cellular morphology, lymphatic invasion, and metastasis to identify what type of tumor each sample was, whether benign or malignant; this was carried out by adhering to the criteria established by the Consensus on Mammary Tumors in Dogs and Cats [
21]. A total of fifty-one canine mammary tissue samples were analyzed and categorized into three groups: (i) the control group, consisting of five samples of normal mammary tissue without neoplasia; (ii) benign tumors, consisting of nineteen samples of benign mixed tumors; and (iii) malignant tumors, which included six grade 1 carcinomas in mixed tumors, five grade 2 carcinomas in mixed tumors, thirteen grade 3 solid carcinomas, one papillary carcinoma, and two tubular carcinomas. All samples were obtained from a veterinary pathology laboratory tumor biobank, ensuring standardized preservation and accessibility for this study. Regarding the test with ZEB1 performed, only 10 tumor samples were used (4 benign and 6 malignant, with the malignant ones subdivided into good and reserved prognosis).
4.2. Positive and Negative Controls
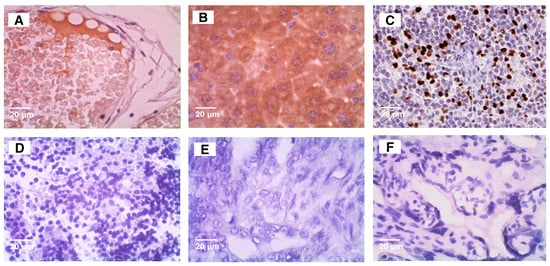
Positive and negative controls for each antibody used (
Figure 1) were obtained according to The Atlas Human Protein website.
4.3. Single Immunohistochemical Labeling
For the single immunohistochemical labeling, 4 μm thick tissue sections were prepared using a microtome from the manufacturer Leica (Model 2255, Wetzlar, Germany). The slides were deparaffinized in xylene and rehydrated through a graded alcohol series, followed by incubation in an oven at 60 °C for 3 h. Antigen retrieval was performed using citrate buffer (pH 6.0) for ITIH2 or EDTA buffer (pH 7.4) for ENO1, both heated to 100 °C in a steam cooker (Histo Bath, from the manufacturer Easy Path, São Paulo-SP, Brazil) for 30 min. Endogenous peroxidase activity was blocked using a DAKO peroxide solution for 15 min, and non-specific protein binding was minimized with a 3% BSA solution in PBS for 10 min.
Primary antibodies (anti-ITIH2 at 1:300, anti-ENO1 at 1:3000, and anti-ZEB1 at 1:500) were applied and incubated overnight at 4 °C to ensure specific binding. Afterward, horseradish peroxidase (HRP)-conjugated secondary antibodies were applied for 25 min. Immunoreactivity was visualized using diaminobenzidine tetrahydrochloride (DAB) as the chromogen (1 drop per 1000 μL of DAKO substrate) for 2 min. Finally, the slides were counterstained with hematoxylin for 2 min to enhance cellular structure visualization and mounted for analysis.
4.4. Double Immunohistochemical Labeling
The double labeling procedure followed the same initial steps as single labeling, including tissue section preparation, deparaffinization, antigen retrieval, and blocking. The distinguishing feature was the sequential application of two primary antibodies. Following the first immunolabeling, which utilized DAB as the chromogen, an acid block was applied to inactivate residual HRP activity and prevent cross-reactivity with the second antibody.
Next, the second primary antibody was introduced, followed by incubation with a distinct HRP-conjugated secondary antibody and visualization using a contrasting chromogen to differentiate it from the first labeling. Stringent washing steps were performed between each stage to remove residual reagents or minimize nonspecific binding.
This method ensures precise localization and differentiation of multiple target antigens within the same tissue section, facilitating a deeper understanding of co-expressed biomarkers.
4.5. Microscopy and Quantification of Immunohistochemical Labeling Intensity
The photomicrographs were captured at 40× magnification on a NIKON ECLIPSE LEICA EC3 microscope, model E200, and using the LAZ EZ Imaging software for Windows, software, version 3.4.0. These images were then analyzed by the IMAGE J [
22], using the IHC Profiler plugin, version 1.46r [
23] that quantified the DAB and hematoxylin staining, generating three numerical results for each sample. First, the software determined the staining intensity by dividing the pixels into three categories: high positive, low positive, and negative. The values obtained were recorded in an Excel spreadsheet, where the following formula was applied to calculate the staining histoscore (HS) 16, 17, which ranges from 0 to 300: HS = (1 × % low positive cells) + (2 × % positive cells) + (3 × % high positive cells).
4.6. Statistical Analyses
Statistical analyses were performed using R [
24,
25] and GraphPad Prism (v. 5.0). The normality of residuals of quantitative variables was analyzed by the Shapiro–Wilk test, and Levene’s test assessed variance homogeneity. Quantitative data were described as mean ± standard error or median with minimum and maximum values, depending on data distribution. Group comparisons were performed using ANOVA followed by Bonferroni post hoc tests,
t-tests with Welch’s correction, or Kruskal–Wallis with Dunn’s post hoc, as appropriate. For categorical variables, Fisher’s exact test was applied. Residuals from the chi-square test were assessed to identify significance in the observed distributions. Count data were modeled using a quasi-Poisson regression. All graphs were generated using the ggplot2 package [
26] and GraphPad Prism (v. 10.3.1).
4.7. Prognostic Classification
The terms “good prognosis” and “reserved prognosis” refer to tumors that, in most cases, tend not to progress to death and those that progress rapidly to death, respectively. For this classification, criteria such as lymphatic invasion, tumor size, morphological aspects of the cells and metastasis were considered.
6. Discussion
The malignant group exhibited a higher mean age compared to the benign group, consistent with findings reported in the literature [
27]. Conversely, tumor size did not differ significantly between the two groups, a result that contrasts with previous studies [
28]. Anatomopathological characteristics such as necrosis, anisoacaryosis, nuclear pleomorphism, and increased mitotic activity were more pronounced in the malignant group, in alignment with established literature [
29].
As a secreted protein, ITIH2 is expected to be present in both normal and tumor tissue, as well as in various locations, including plasma within vessels and in the extracellular medium. This widespread distribution complicates the establishment of a direct relationship between ITIH2 and breast tumors, as it is observed in all samples across groups. To overcome this challenge, a specific staining criterion was adopted: staining was considered positive only when present within tumor cells. In our findings, no intense ITIH2 staining was detected within cells in any sample. Moderate to weak cytoplasm staining was observed in some cases, with rare nuclear staining. This weak intracellular presence suggests that there is another function of ITIH2 besides its primary one, in the extracellular matrix and plasma. However, its occasional intracellular detection warrants further investigation into what its potential additional roles are, especially in cancer biology. Despite these results highlighting a limited cellular presence of ITIH2, its role in inflammation and extracellular matrix stabilization suggests potential relevance to breast cancer prognosis [
30]. ITIH2 appears to have a dual, context-dependent function in tumor biology. On the one hand, its antiproteolytic function and regulation of the extracellular matrix (ECM) may suppress tumor progression. On the other hand, interactions with molecules such as hyaluronic acid and modulation of the inflammatory microenvironment may exert ambivalent effects, depending on the tumor’s stage and context. A comprehensive understanding of ITIH2’s role in tumors requires considering the specific cancer type, disease stage, and the surrounding microenvironment.
The expression of ITIH2 in breast tumors can be explained by its binding with hyaluronic acid, a molecule overexpressed in malignant tumors. Interestingly, hyaluronic acid serum levels do not increase in patients with localized breast tumors but rise during advanced disease stages [
31]. In contrast, studies have shown reduced hyaluronic acid levels in infiltrating breast tumors [
32,
33]. Hyaluronic acid cross-linking with ITIH2 via tumor necrosis factor-induced protein 6 (TSG6) reduces its degradation and alters extracellular matrix (ECM) interactions, which may offer cancer-protective effects [
34]. Although our discussions on ITIH2 are primarily based on studies of human breast cancer, this is due to the lack of specific data for dogs. This gap underscores the need for future investigations exploring the role of this protein in canine mammary tumors, contributing to a better understanding of its relevance in this context.
Regarding ENO1, the fact that there were no differences between the groups draws attention to the specificity of the antibody used. A higher frequency of simultaneous cytoplasmic and nuclear staining was observed in the same cell in all groups, especially in the control group, which exclusively presented this type of staining; in the benign group, there was mostly simultaneous staining of the cytoplasm and nucleus, but there was also staining only in the cytoplasm in some samples; in the malignant group, there was a lot of simultaneous staining of the cytoplasm and nucleus, but in some samples it stained only the cytoplasm, and in others it stained the plasma membrane. Cytoplasmic staining is in line with its role as a glycolytic enzyme, while nuclear localization—reported predominantly in benign tumors—is linked to its isoform MBP-1, which binds to C-myc, inactivating it, thus acting as a tumor suppressor [
35]. However, cytoplasmic staining does not always predict ENO1, and it may be MBP1, its isoform.
In malignant cells, there was simultaneous staining of the cytoplasm and nucleus, as well as staining of the cell membrane, which did not occur in the benign group or in the control group. This fact corroborates studies that report the expression of ENO1 on the cell surface exceptionally in malignant tumors, where it binds to plasminogen to promote ECM degradation and metastasis [
36]. Our findings using the anti-ENO1 antibody revealed expression of both ENO1 (cytoplasm/membrane) and MBP1 (nucleus and cytoplasm). A study showed that both proteins can be expressed in normal and tumoral mammary glands [
37]. Our results show that in all groups there was a majority staining of the nucleus and cytoplasm, with additional expression of the membrane, exclusive to the malignant tumor group.
This shows that concomitant staining of ENO1 and MBP1 may have occurred in all groups, but we were unable to differentiate them because we used an antibody that recognizes ENO1 and its isoform MBP1. We interpret this as stating that it is necessary to use antibodies specific for ENO1 and another for MBP1; only then could we be certain of the quantifications related to each one, as well as knowing which of the two isoforms would be in the cytoplasm, since both can be expressed in the cytoplasm. Corroborating this, an article [
37] stated that the studies conducted so far in tumor and normal tissue samples depend on the use of commercially and non-commercially available antibodies recognizing both ENO1 and MBP-1, which has made it difficult to clearly assess the relative expression of the two proteins by immunohistochemistry. We hypothesize that if there is greater expression of ENO1 to the detriment of MBP1, this could evidence energetic favoritism for the tumor; on the other hand, if there is majority expression of MBP1, it could be inferred that the tumor cells are in hypoxia, since MBP1 does not have an enzymatic function in glycolysis like ENO1. As a conclusion regarding the expression of ENO1, we can state that we are certain of its membrane expression exclusively in malignant tumors.
The low AUC values indicate that ITIH2 and ENO1 have limited predictive capability. The wide confidence intervals and lack of statistical significance further highlight their limited utility as individual prognostic markers. These findings underscore the need for larger studies to confirm these results and suggest that combining biomarkers or exploring alternative analytical approaches may yield better predictive performance. Further research into the biological roles of ITIH2 and ENO1 is also warranted.
In canine mammary tumors, foreign tissues such as bone and cartilage are commonly observed due to epithelial–mesenchymal transition. Knowing that ZEB1 regulates the transcription of ITIH2, the additional test we performed with ZEB1 aimed to investigate its presence for two reasons: the first was to find out whether there is a relationship between ZEB1 and ITIH2 in canine mammary tumors, and the second was to confirm whether ZEB1 regulates the transcription of ITIH2 [
38]. Our results regarding the immunohistochemical test with ZEB1 and visual analysis showed that this protein is indeed present in both benign and malignant mammary tumors of bitches. However, it is important to clarify that statistically the number of samples, both total and distributed in the subgroups “good prognosis and reserved prognosis”, was insufficient. However, our objective was only to collaborate with future studies, verifying the absence or presence of ZEB1 and whether this coincided with the intensity of staining of ITIH2 and ENO1, as well as the absence/presence of these two proteins, all this to suggest future studies of colocalization/co-expression of these proteins in mammary tumors of bitches. It was observed that in tumors with good prognosis, ITIH2 presented absent to moderate staining, while ZEB1 presented intense staining in most samples, although absent in a small part; in tumors with reserved prognosis, ITIH2 did not differ in its staining pattern, but ZEB1 stained intensely and strongly in all samples.
In double staining with ENO1 and ZEB1, we observed that in tumors with good prognosis, while ENO1 was marked strongly in most samples, ZEB1 was absent. In tumors with reserved prognosis, it was observed that ZEB1 was present with strong intensity in all samples. Moreover, ENO1 was also present in all samples, but was ranging from moderate to strong intensity. In summary, in mammary tumors of dogs, ZEB1 was found in tumors with good prognosis and in those with poor prognosis but showed a tendency to be present and to mark more intensely in tumors with poor prognosis; in addition, its expression showed no association with ITIH2, but it did with ENO1.
CD44 is a transmembrane glycoprotein that acts as an endocytic HA receptor in human breast tumor cells [
39]; therefore, future investigations should explore the interactions between ITIH2, hyaluronic acid, and CD44 in canine and human breast tumors, given the evidence of potential functional relationships. Furthermore, ZEB1 could also be included in these investigations.
Regarding ENO1, future studies with larger sample sizes are essential to elucidate its interaction with ZEB1 and its subcellular localization in tumor cells, as these factors provide significant information on tumor progression and prognosis.