1. Introduction
Osteoarthritis (OA) is a chronic rheumatic disease that primarily affects the joints, particularly the knees, hands, hips, and spine [
1]. As the most common musculoskeletal disorder, it results in significant loss of function and quality of life [
2]. Pain in OA is closely tied to physical function: movement generates pain, which in turn limits physical activity. Joint stiffness, especially in the morning, and a sensation of instability are also common symptoms [
2]. In addition, persistent pain at rest or at night may be a sign of advanced osteoarthritis [
3]. While osteoarthritis has traditionally been considered a degenerative and wear-and-tear process, its pathogenesis is now understood to be much more complex, with the suffix “-itis” clearly indicating an inflammatory process [
1,
3,
4].
Age is one of the most important risk factors. The vulnerability of the joint occurs as an ageing process that makes it susceptible to disease. Thus, the incidence in people under 40 years of age is low and generally associated with trauma. Prevalence increases between the ages of 40–60 years. It is estimated that 9.6% of men and 18% of women worldwide have symptomatic osteoarthritis [
2]. In men aged 60–64 years, it is more frequent in the right knee (23%) than in the left (16.3%), while in women, the distribution seems to be more balanced [
5].
In relation to gender and race, there are some differences. Women have a higher risk of developing osteoarthritis than men. Regarding race, African American and Chinese women have a higher frequency of osteoarthritis of the knee, differences that could be attributed to a genetic but also environmental component, related to the joint overload associated with the more common work activity in these groups [
6]. Other very important risk factors are obesity or being overweight due to an increase in the mechanical forces on the joints that support our weight, and this is probably the primary factor that contributes to accelerating the degenerative process [
2].
Conventional pharmacological treatments for OA, such as nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids, primarily target inflammation to alleviate symptoms, but they do not alter the progression of the disease. Moreover, both pharmacological and surgical approaches often present challenges, including invasiveness, significant costs of the procedures, and a notable risk of adverse effects [
1,
7]. This underscores the necessity for alternative, conservative therapies for OA that are grounded in evidence-based mechanisms of effectiveness. In this context, spa therapy consists of multiple techniques based on the healing effects of water, including hydrotherapy, balneotherapy, and mud therapy, often combined with therapeutic exercises, massage, or physical therapy [
8,
9,
10,
11,
12].
Balneotherapy, either alone or in combination with mud (mud therapy or pelotherapy), is one of the most widely used non-pharmacological complementary treatments for various rheumatic diseases, including osteoarthritis (OA). Its beneficial effects likely stem from a combination of mechanical, thermal, and chemical actions. Balneotherapy has been shown to alleviate pain and stiffness, enhance joint mobility and improve quality of life. Most importantly, it may also play a role in preventing or delaying the progression of OA [
13]. A growing body of evidence supports the therapeutic benefits of balneotherapy [
14,
15,
16,
17], particularly mud therapy, on function, perceived pain, and quality of life in individuals with OA. These findings suggest that these therapies are effective and safe options for the clinical management of this condition [
13,
18,
19]. This effect may be particularly due to its anti-inflammatory, antioxidant, and chondroprotective properties [
20,
21]. Therefore, fortifying peloids used in balneotherapy with compounds that enhance their antioxidant and anti-inflammatory properties, such as polyphenolic acids, could represent a promising strategy to augment the clinical effectiveness of mud therapy interventions.
In general, polyphenolic acids found in plants are being used as natural products with potential and proven health benefits. Among these, rosmarinic acid (RosA), although originally identified and isolated from
Rosmarinus officinalis L., is present in numerous species of the Lamiaceae family, where it has been reported to exhibit a wide range of biological activities, including anti-inflammatory, antioxidant, antidiabetic, antiviral, and antitumor properties, among others [
22]. Due to its potential anti-inflammatory capacity, RosA has become a valuable natural resource for individuals suffering from chronic diseases such as arthritis or osteoarthritis, as inflammation and joint wear contribute to pain and reduced mobility. While some studies evaluating its clinical benefits have utilised RosA emulsions [
23] or administered it orally [
24], most of its applications have been assessed within the field of food sciences [
22], particularly regarding the symptoms of osteoarthritis [
25,
26]. However, there are no studies in the scientific literature regarding the use of RosA in the context of balneotherapy, either (pelotherapy or mud therapy) with or without peloids.
Given the industrial interest in systematically maturing peloids to enhance the consistency of their production and composition throughout the year, while also improving their organoleptic properties, this study aimed to evaluate whether balneotherapy with a controlled-matured peloid, with or without fortification with RosA enhances clinical and functional outcomes. Specifically, clinical symptoms (pain measured using the Visual Analogue Scale, VAS), objective functional measures (assessed by knee flexion and extension), perceived functional status (evaluated using the Western Ontario and McMaster Universities Osteoarthritis Index, WOMAC), and quality of life (measured with the EUROQOL questionnaire) were examined in elderly OA patients after mud therapy interventions with or without RosA. Furthermore, to assess immunological safety and avoid an innate immunocompromised status due to the balneological interventions, the phagocytic and microbicidal capacities of neutrophils were evaluated, and the effects of the RosA-fortified peloid were compared with those of the non-fortified version.
This pilot study, conducted within the IMSERSO thermal therapy programme in Spain for elderly individuals, represents the first investigation into the clinical effects (symptomatic and functional) of RosA in the context of balneotherapy. Understanding these effects, as well as ensuring the safety of its application—including the absence of immune compromise in participants—is crucial before undertaking larger studies that explore potential new mechanisms of action.
2. Materials and Methods
This prospective, triple-blind, controlled study was conducted at the healthcare and spa centre “El Raposo” (Puebla de Sancho Perez, Badajoz, Spain), which was declared a Public Utility in 1926. The effects of a cycle of mud therapy were evaluated using artificially matured mud, with or without fortification with RosA, in a group of patients with OA participating in the Social Thermalism Programme organised by the Elderly and Social Services Institute (IMSERSO) (Spain).
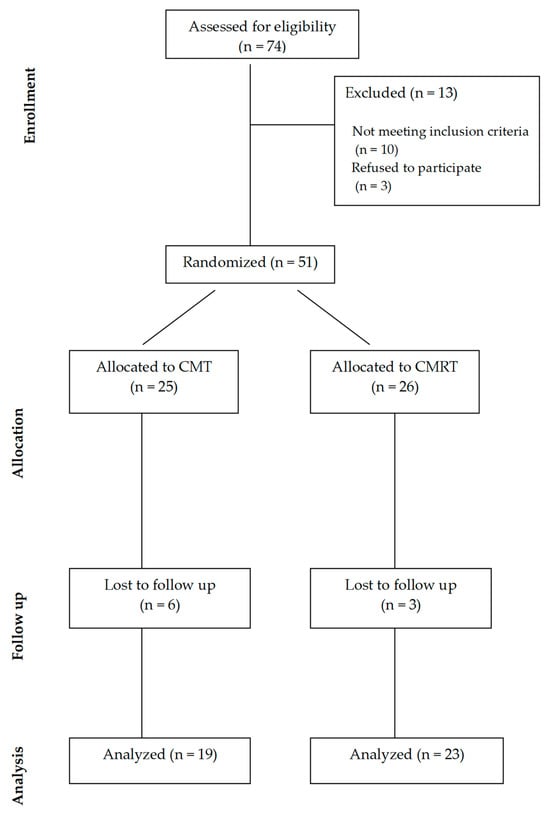
Seventy-four patients with primary knee osteoarthritis were evaluated for eligibility in the study. After being informed of the research, all volunteers from this homogenous group who met the inclusion and exclusion criteria were accepted. The inclusion criteria required participants to be ≥60 years old and to have a diagnosis of primary knee OA by a rheumatologist according to the American College of Rheumatology (ACR) criteria [
27]. Additionally, participants must not have any diagnosed conditions that contraindicate experimental treatment, and they must possess sufficient cognitive ability to respond accurately to the questionnaires assessing the health variables under investigation. All volunteers underwent an initial medical evaluation to identify any potential contraindications to the experimental treatment. According to the pre-intervention knee flexion angle, no volunteers exhibited very severe symptoms of OA.
The exclusion criteria were having any infection, neoplastic illness, or cardiopulmonary, vascular, inflammatory, immune, or other musculoskeletal condition, having undergone total or partial knee replacement, having received physical therapy in the previous six months, having taken NSAIDs in the previous three days, having received intra-articular injections of corticosteroids or hyaluronic acid in the previous six months, or having received oral or local corticosteroid or anticytokine or any biological therapy. A total of 51 patients were included in the study after meeting the eligibility criteria, with 26 of them receiving the controlled mud therapy (CMT) intervention and 25 receiving controlled mud fortified with RosA (CMRT) therapy. Nine patients were lost to follow-up, leaving forty-two patients for evaluation (
Figure 1).
This sample sise of the present pilot study allowed us to obtain clinically, physiologically, and statistically reliable differences, also taking into account ethical criteria, cost, and feasibility.
Table 1 shows the anthropometric data of the final volunteers participating in the study.
Informed consent was provided by all patients before being included in the study. Each participant was identified with an alphanumeric code in order to preserve their anonymity and to conceal from researchers which treatment group each patient was assigned to. Furthermore, both muds were identical in appearance and prepared in numeric-code-labelled buckets, so neither the patient nor the physiotherapist could identify them. Anthropometric parameters and other clinical variables were evaluated along with blood sampling between 8.00 a.m. and 9.00 a.m. Baseline evaluation and sampling were performed one day after arrival to the spa centre, before initiation of the first session of mud therapy. Post-treatment sampling was carried out a day after the last session of mud therapy, thus avoiding the evaluation of the effect of an acute intervention. This study was approved by the Ethical Committee of the University of Extremadura, Spain, in accordance with the guidelines of the European Community Council Directives and the Helsinki Declaration (Nº Reg. 96/2022 and Nº Reg 146/2024).
2.1. Intervention
The spring water of the spa centre “El Raposo” emerges at 16.5 °C and forms a stream where the mud is collected. In order for it to mature naturally, it is placed outdoors in a tank containing the mineral-medicinal water, and it is left for 5 to 8 months, when it acquires optimal biological, chemical, and thermophysical properties for application. However, artificially matured mud is a solid/liquid content mixture at controlled proportions, in order to obtain a final product with the same basic properties as the natural mud, which has clear industrial interest to enhance the consistency of their production and composition throughout the year, also improving their organoleptic properties. “El Raposo peloid” is composed of a mixture of bicarbonate mineral–medicinal water, a clayey-silt solid phase, implemented with a microalga and, potentially, with rosmarinic acid. Biological content (microalgae, Monoraphidium pusillum, identified by Spanish Bank of Algae) is obtained from the natural spring and cultivated separately using photobioreactors and later added to the mixture also at controlled proportions (1%). In this way, controlled mud takes at least two months to complete maturation. Finally, after maturation of peloid, 0.5% of rosmarinic acid (Sigma-Aldrich, Burlington, MA, USA, CAS number 20283 92-5), as bioactive compound, was added.
The water of “El Raposo” spa is hypothermal, very hard, of medium mineralisation, and contains bicarbonate (396.5 mg/L) and calcium (130.2 mg/L) as predominant ions. The mineral–medicinal water fraction represents a 32% approximately of the mud. The muds’ solid content (65% approximately) consists of a mixture of silt, clay and sand. They are basically composed of phyllosilicates (smectite and illite), quartz and calcite. The major chemical elements of the muds are SiO
2, CaO, Al
2O
3 and Fe
2O
3 [
28,
29].
Patients of both groups participated in daily mud therapy sessions for 10 consecutive days, in line with the therapeutic protocol prescribed by their healthcare professionals. The intervention was identical for both groups with the only difference being the type of mud, and it was always conducted in the morning by the same physiotherapists. The sessions consisted in the whole-body application of mud at 40–42 °C by brush, followed by a drying period of 45–60 min in a solarium. Subsequently, patients received a mud bath (consisting of mineral-medicinal water and mud) at 38–40 °C for 15 min. Finally, mud remnants were removed with a thermal water jet (38–40 °C) for 2 min. It is noteworthy to mention that whole-body mud application by brush is a particularly characteristic technique carried out in this spa centre. There were no adverse events.
2.2. Anthropometric and Clinical Data
Body weight, body mass index (BMI), and knee flexion and extension angle were measured by standardised methods. Thus, the BMI was calculated using the formula: BMI = weight (kg) height2 (m2), and knee flexion and extension angles were determined using a goniometer.
Perceived pain intensity was assessed using a visual analogue scale (VAS) [
30]. Moreover, Western Ontario and McMaster Universities Arthritis Index (WOMAC) was used to evaluate OA-related pain, stiffness, and physical function [
31]. The EuroQol-5D (EQ-5D) questionnaire was used to evaluate the health-related quality of life [
32]. Beyond the total score, and to assess potential differences in more specific aspects of the WOMAC and EQ-5D questionnaires, the individual dimensions or health domains comprising each questionnaire were also analyzed separately. In the case of the WOMAC, the dimensions related to “pain”, “morning stiffness”, “stiffness during the rest of the day”, and “difficulty in performing daily activities” were assessed and compared individually between the two peloids. For the EQ-5D, the domains of “mobility”, “self-care”, “activities of daily living”, “pain and discomfort”, and “anxiety/depression” were similarly evaluated and compared separately between the two peloids.
2.3. Whole Blood Extraction and Evaluation of Neutrophil Phagocytic and Microbicidal Capacity
Peripheral blood samples were collected in a fasted state through sterile puncture of the antecubital fossa veins and placed in heparinised tubes. The phagocytic and oxygen-dependent microbicidal capacity (production of O2−) of neutrophils against opsonised Staphylococcus epidermidis was assessed using flow cytometry on heparinised whole blood. This quantitative method enables the evaluation of the percentage of neutrophils with phagocytic and microbicidal capacities.
Briefly, bacteria were stained with fluorescein isothiocyanate (FITC) at a concentration of 1 μg/mL and opsonised with human serum. Blood from each patient (200 μL) was incubated (1 h, 37 °C in the dark with shaking) in the presence of 50 μL of opsonised bacteria, 10 μg/mL of Hoechst 33342, 1 μg/mL of 7-actinomycin D (7AAD), 250 μL of PBS, and 2% fetal bovine serum (FBS). After the initial 30 min of incubation, hydroethidine (HE), a specific probe used to detect intracellular superoxide anion production by NADPH oxidase, was added, and the mixture was incubated for an additional 30 min. The control samples consisted of 100 μL of blood mixed with 10 μg/mL of Hoechst 33342, 1 μg/mL of 7AAD, 400 μL of PBS, and 2% FBS. Blood samples were subsequently analyzed using a flow cytometer (MACSQuant
® VYB, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) [
33].
2.4. Statistical Analysis
The data are presented as the mean ± standard error of the mean (SEM). The normality of the variables was assessed using the Kolmogorov–Smirnov test, followed by Student’s t-test for paired samples in the pre-/post-intervention study for each peloid. A two-way ANOVA was used to evaluate statistical differences between the effects of the two peloids on variables exhibiting different behaviours. The significance level was set at p < 0.05. Calculations were performed using IBM SPSS Statistics version 22 software.
4. Discussion
In medical hydrology, balneotherapy, either alone or in combination with mud (mud therapy or pelotherapy), is one of the most widely used non-pharmacological complementary treatments for various rheumatic diseases, including osteoarthritis (OA). The therapeutic effectiveness of this therapy in OA has been well-established through several studies [
10,
12,
21,
34,
35], including those conducted by our research group at the same spa centre as in the present investigation, El Raposo, using naturally matured peloids [
36,
37]. The results of the present study also demonstrate the clinical and functional benefits of artificially matured peloids in a controlled manner. Furthermore, it was observed that when the peloids were fortified with RosA, in addition to these clinical and functional improvements, there was a stimulation of the innate immune response in patients with OA. This is the first study to evaluate the effect of RosA in the context of balneotherapy.
Peloids are a well-tolerated thermal treatment commonly used for sports injuries and rheumatic diseases, particularly osteoarthritis (OA). Peloids consist of a solid phase, a liquid phase, and a biological phase. The solid phase is primarily composed of clays, peat, or silt, for example, while the liquid phase consists of mineral–medicinal water from thermal medicinal springs (sometimes including seawater or salt-lake water). The biological phase generally includes microalgae and/or cyanobacteria. While the solid phase mainly contributes to heat exchange, the liquid phase may play a significant role in the anti-inflammatory properties of the peloid, and the biological phase can provide compounds with therapeutic biological properties [
38]. In this context, peloids have been defined as “substances formed in nature through geological processes, which, when finely granulated and mixed with water, have applications in medical practice in the form of baths or poultices” [
39], a definition adopted by the International Society of Medical Hydrology in 1938 [
40].
Previous studies from our group at the “El Raposo Thermal Medical Balneario”, using peloids naturally matured in the stream through which the mineral–medicinal water flows, demonstrated clear functional and symptomatic benefits in elderly individuals with osteoarthritis (OA). These benefits are thought to result from the stabilisation of immunoneurondocrine interactions, balancing inflammatory and physiological stress responses as mechanisms of effectiveness [
20,
21,
36,
41,
42,
43]. However, for any balneario business, it is both industrially and hygienically important to be able to control the maturation of its peloids, ensuring better production consistency throughout the year while maintaining sanitary safety. Therefore, the aim of this study was to evaluate the effects of a peloid with similar solid, liquid, and biological phases, but artificially and systematically matured. Furthermore, given that many of the clinical functional and symptomatic effects of pelotherapy are attributed not only to hyperthermia and the chemical components of the liquid phase but also to the effects induced by the biological phase, we aimed to explore whether the incorporation of RosA in this peloid could enhance the clinical benefits.
The results herein presented corroborate the clinical benefits of mud therapy in elderly OA patients, even with an artificially controlled maturation of the peloid. This was manifested in improvements in knee flexion and extension angles, perceived pain, stiffness, functional capacity, and health-related quality of life. These clinical benefits observed after the cycles of mud therapy with artificially controlled maturation of peloids are at least as effective as those observed in previous studies with naturally matured peloids and the same application techniques at the same medicinal spa [
42], as well as in other balneological interventions using local medicinal mud packs to evaluate similar parameters [
44].
It is important to note that, given its comparable clinical effects, the controlled maturation of peloids may offer the balneotherapy industry not only greater control over production and composition, which would be less dependent on annual climatic variations, but also the opportunity to integrate the biological phase with additional components possessing potential therapeutic properties. In this context, RosA, due to its natural anti-inflammatory properties in pain reduction [
22], has been proposed as a therapeutic adjunct in chronic conditions, such as osteoarthritis (OA). However, although these properties have been explored using RosA emulsions [
23] or, more broadly, in food sciences [
22,
26], to the best of our knowledge, this is the first study to evaluate the role of RosA in balneotherapy with peloids or mud therapy. The addition of RosA to the artificially matured peloid also resulted in improvements across all clinical variables assessed in this study, further confirming the effectiveness of this balneological intervention. Notably, only the OA patients receiving the peloid with the biological phase enriched with RosA showed improvements in anxiety/stress as a dimension of health, suggesting a potential enhancement of the therapy for future studies and interventions in OA patients, which may also have a beneficial impact on their mental health.
Once the safety of applying peloids containing RosA has been established, with no allergic reactions or other visible side effects observed and considering that some of the effects attributed to RosA are related to the immune system, we focused on evaluating its impact on the innate immune response. This is particularly relevant in OA patients, who exhibit a reduced phagocytic capacity of neutrophils [
33]. Additionally, given that the hyperthermia-induced stress from the intervention could potentially lead to transient immunosuppression in elderly individuals, understanding these immune responses is crucial. Surprisingly, the results clearly indicate that while controlled mud therapy without RosA intervention did not affect the percentage of neutrophils with phagocytic capacity and reduced those with oxygen-dependent microbicidal capacity, the controlled mud therapy with the RosA-fortified peloid significantly stimulated both phagocytic and microbicidal capacities, enhancing the ability of elderly OA patients to combat pathogen challenges. This supports an additional benefit induced by the incorporation of RosA into the biological phase, further demonstrating a novel beneficial effect of RosA on the immune system when applied in pelotherapy.
In conclusion, this pilot study demonstrates that mud therapy interventions using peloids artificially matured in a controlled environment and composition offer both industrial and clinical benefits in the context of balneological treatments for OA patients. In addition to the similar functional and symptomatic benefits, the main clinical relevance of incorporating RosA into the biological phase of the peloid is that, in contrast to the peloid without RosA, it enhances the innate immune response to pathogen challenges. This could potentially compensate, at least in part, for the deficiencies in immune response reported in elderly OA patients.
Limitations of the Study
Given that there was no prior knowledge regarding the effects of incorporating RosA into artificially matured peloids for use in mud-bath therapies, this work represents only a pilot study with a limited sample of elderly patients with moderate osteoarthritis (OA), focusing on the clinical and functional benefits affecting their quality of life and innate immune response. However, this study did not delve into the immunophysiological mechanisms underlying its effectiveness. Therefore, once the effectiveness and safety of these interventions in elderly patients are confirmed, further research will aim to explore their anti-inflammatory and immunoneuroendocrine regulatory effects.