1. Introduction
Head and neck cancers are predominantly squamous cell carcinomas (SCC). However, tumors affecting different regions within this category can vary significantly in terms of invasiveness, growth rate, and metastatic potential [
1]. To better understand the behavior of these tumors, researchers worldwide have been increasingly focusing on biomarker studies over the past few decades. Biomarkers play a critical role in improving the precision and effectiveness of cancer diagnosis, prediction of treatment response, and management [
2].
In this study, we systematically examined the expressions of several published prognostic markers (Ki67, p53, EGFR, COX-2, Cx43) and p16 in head and neck squamous cell carcinomas from various anatomical regions using immunohistochemistry.
Reports indicate that HPV (human papillomavirus)-positive oropharyngeal SCC represents a distinct clinicopathological entity with a better prognosis compared to HPV-negative oropharyngeal SCC [
3]. Multiple studies have shown that increased expression of p16 is an excellent surrogate marker for HPV-positive oropharyngeal SCC [
4].
Ki-67 is a protein that is associated with cell proliferation. High Ki-67 levels in head and neck cancer are generally associated with more aggressive tumors and poorer prognosis. Sometimes, Ki-67 is used as part of the grading system for tumors, helping to guide treatment planning [
5,
6].
The p53 protein is crucial for DNA stability and cancer prevention. Its role in head and neck cancer is complex and context-dependent. As a tumor suppressor, p53 regulates the cell cycle, apoptosis, and genomic stability. High levels of normal p53 can indicate a positive prognosis by promoting apoptosis and DNA repair, aiding in cancer control and enhancing treatment effectiveness. Conversely, high p53 levels due to TP53 gene mutations result in stable, mutant p53 proteins, leading to uncontrolled growth, resistance to apoptosis, and genomic instability, often associated with more aggressive cancer and worse outcomes, including in HNSCC [
7,
8].
Epidermal growth factor receptor (EGFR) is a protein that, when overexpressed or mutated, can play a significant role in the development and progression of various cancers. High EGFR levels in HNSCC indicate poor prognosis, as EGFR promotes tumor growth, invasion, and metastasis. It serves as both a prognostic marker and a therapeutic target. EGFR inhibitors, such as monoclonal antibodies and tyrosine kinase inhibitors, are critical in treatment [
9].
Cyclooxygenase-2 (COX-2) is an enzyme involved in the synthesis of prostaglandins, which play a role in inflammation and cell proliferation. COX-2 overexpression is associated with enhanced tumor growth, angiogenesis, and metastasis. Prostaglandins produced by COX-2 can promote these processes, contributing to the aggressiveness of the cancer. It is also considered both a prognostic marker and a potential therapeutic target in head and neck squamous cell carcinoma (HNSCC) [
10].
Membrane connexin 43 (Cx43) forms gap junctions, which are channels enabling direct communication between adjacent cells. Experimental data indicate that gap junction proteins play a significant role in tumor growth and progression. These intercellular channels, composed of about 21 connexins, facilitate metabolic cooperation, growth, and development. The absence of gap junctions and intercellular communication can contribute to tumorigenesis by promoting cell migration, invasion, and metastasis [
11]. Of the 21 connexins, Cx43 is the most well-known and extensively studied. It is broadly expressed in epithelial cells, hematopoietic cells, neurons, astrocytes, cardiac neural crest cells, and fibroblasts. Cx43 regulates cell proliferation and apoptosis by forming hemichannels that exchange growth and apoptotic factors, while also promoting tumor progression and metastasis. Recent studies suggest that Cx43 plays a more complex role in various stages of tumor progression [
12,
13]. Assessing Cx43 expression can help determine the aggressiveness of cancer, guide treatment strategies, and explore targeted therapies that could potentially restore its function or enhance its tumor-suppressive properties. Measuring Cx43 levels in tumor tissues could also have potential diagnostic use [
14,
15].
Aim: Our objective was to investigate how regional variations in the expression of potential biomarkers of SCC progression in the head and neck region contribute to understanding the diverse behaviors of these malignancies and to correlate results with clinicopathological parameters.
2. Materials and Methods
We performed immunohistochemistry on 91 histologically verified HNSCC cases [48 laryngeal (L), 25 extralaryngeal (E), 18 cases affecting multiple regions (E-L)] from 2010 to 2016 selected from the archive of the County Emergency Hospital, Targu Mures. The laryngeal group (L) included HNSCC from supraglottic, glottic, or subglottic areas; the extralaryngeal group (E) covered tumors in the nasopharynx, oropharynx, hypopharynx, oral cavity, and mobile tongue; and the mixed group (E-L) comprised cases with both laryngeal and extralaryngeal spread. We classified patients’ squamous cell carcinomas into three groups: classic (CSCC), variants (CSCV—basaloid, spindle cell, acantholytic, verrucous, lymphoepithelial, papillary, and adenosquamous), and mixed-type (CSCM—tumors with features of two histological types). CSCC included keratinized and non-keratinized tumors. We used the WHO-recommended three-tier grading system to grade tumors: G1 for well-differentiated, G2 for intermediate, and G3 for poorly differentiated tumors.
In the Immunohistochemistry Laboratory of the Department of Anatomy and Embryology at the “George Emil Palade” University of Medicine, Pharmacy, Science, and Technology, the 3 μm thick sections obtained from the formalin fixed and paraffin embedded resection tissue specimens were dewaxed and rehydrated followed by endogenous peroxidase blocking. Antigen retrieval was performed by pressurized steam cooking (citrate solution, pH 10 for p53, pH 6 for Cx43) or microwave treatment (pH 10 forKi67, EGFR, pH 6 for p16, COX-2). We used mouse monoclonal antibodies for p53 (clone DO-7, Novocastra Laboratories Ltd., Leica Biosystems, Deer Park, IL, USA) in 1/800, Ki67 (clone MM1, Novocastra Laboratories Ltd., Leica Biosystems, Deer Park, IL, USA) in 1/150, EGFR (clone EGFR.113, Novocastra Laboratories Ltd., Leica Biosystems, Deer Park, IL, USA) in 1/40, COX-2 (clone COX229, Thermo Fisher Scientific, Waltham, MA, USA) in 1/100 for 1 h, as well as Cx-43 (clone CX-1B1, Thermo Fisher Scientific, Waltham, MA, USA) in 1/100 (overnight, 4 °C). For p16, we applied rabbit clonal antibody (clone R19-D, DB Biotech, Inc., Kosice, Slovakia) in 1/200 (1 h). For signal amplification, the secondary antibody EnVision Flex/HRP (Horseradish peroxidase) (Dako, 20 min) was used. Detection of primary antibodies was achieved using 3,3′-Diaminobenzidine (DAB, Dako, Santa Clara, CA, USA). The slides were then counterstained with hematoxylin, dehydrated, and mounted. Negative controls were performed by omitting the primary antibody. Immunohistochemical reactions were read by an independent pathologist.
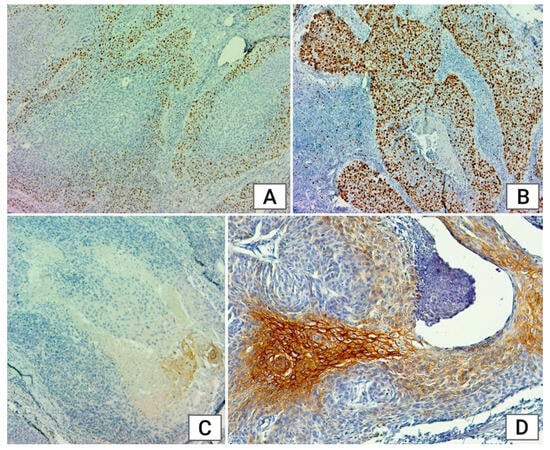
Nuclear expression was observed for p53 and Ki67 (only the infiltrative component on 1000 cells), while COX-2 displayed cytoplasmic expression. The expression was membranous for EGFR and Cx43. We interpreted the results for Ki67, COX-2, and Cx43 according to the following immunoexpression scoring system: S1: 0–10%, S2: 1–25%, S3: 26–50%, S4 > 50%. For p16, we classified the reaction as positive if at least 75% of the infiltrative tumor cells showed intense cytoplasmic and nuclear positivity, considering the remaining reactions as negative.
We classified p53 into two categories: mutant and non-mutant (wild-type). Three types of reactions were considered as mutants: negative nuclear reaction (no cells with a positive nuclear reaction), positive nuclear reaction (more than 90% of tumor cells present an intense, uniform nuclear reaction), and cytoplasmic reaction (tumor cells present an aberrant positive cytoplasmic reaction and not nuclear), while all other reactions were classified as wild-type.
EGFR was scored based on membrane staining intensity: 0 = no staining or <10% of tumor cells show membrane staining; 1+ = faint membrane staining in >10% of tumor cells; 2+ = moderate membranous staining for at least 10% of tumor cells; 3+ = >10% of tumor cells with strong membranous staining.
For statistical analysis, we used GraphPad InStat 3 software, version 3.06 (GraphPad Software Inc., San Diego, CA, USA). A significant association was taken into consideration at a p value of <0.05, with a 95% confidence interval.
Ethics. This study was approved by the ethics committee of “George Emil Palade” University of Medicine, Pharmacy, Science, and Technology of Târgu Mureș, Romania (no. 3211/10/06/2024, provided on 10 June 2024).
4. Discussion
The incidence of head and neck cancers continues to show an increasing trend worldwide. In our study population, 95% of the patients were male, with most aged between 51 and 60 years; although, the number of female patients is also rising [
16]. HNSCCs are a diverse, aggressive, and genetically complex group of malignancies. For decades, the prognosis of tumors and their expected response to different treatments have mainly been predicted using the TNM classification. In recent years, many studies have explored the importance of various immunohistochemical markers and their clinical utility in offering more precise and personalized prognoses.
Across the range of markers studied, one of the most promising is EGFR, with its overexpression or aberrant activation potentially leading to uncontrolled cell growth and tumor development [
17]. We observed milder EGFR expression in both younger and older patients, while those aged 50–70 exhibited higher expression levels, which may indicate more aggressive tumor growth and a poorer prognosis in this age group, as reflected in the literature [
3,
18].
In the analysis of all factors, low p16 expression was observed in most tumors, not reaching the positivity criteria in any case. This largely aligns with the literature, which indicates that p16 positivity is less likely to be expected of tumors that originated from or involved the larynx. Additionally, none of the tumors diagnosed in extralaryngeal regions showed positive p16 expression [
19,
20,
21].
Ki67 levels provide insights into cancer proliferative activity and significantly correlate with tumor grade (
p = 0.048). Well-differentiated tumors showed minimal Ki67 expression, intermediate-grade tumors mostly exhibited S1 expression (51.42%), and poorly differentiated tumors predominantly had S3 expression (35.48%). This finding supports existing literature, which indicates that higher Ki67 expression is associated with a greater likelihood of poorly differentiated, poorer-prognosis tumors [
6]. We observed higher Ki67 expression in extralaryngeal tumors compared to tumors originating from or involving the larynx, with a significant correlation based on tumor localization (
p = 0.01).
Examining p53 expression in relation to various factors, we observed that mutant p53 expression was prevalent in most cases, indicating a worse prognosis. Specifically, younger patients (under 60 years) had a higher ratio of tumors with mutant p53 expression. This correlation with age was statistically significant (
p = 0.02), suggesting that tumors in younger individuals may be more aggressive and associated with a worse prognosis, reflecting a more aggressive tumor phenotype. The gender distribution analysis revealed that all tumors in women exhibited mutant p53 expression, indicating a worse prognosis compared to men and suggesting the need for more complex therapeutic solutions. The majority of cases with mutant p53 expression had negative mutant reactions with 0% cell staining. Currently, the literature does not emphasize whether mutant p53 expression is classified as negative or positive. Wild-type p53 expression was observed only in histopathologically mixed-type tumors, suggesting that tumors with the features of two histological types may be more aggressive [
22].
No significant differences in COX-2 expression were observed across different tumor localizations, with most anatomical regions showing S3 expression, which means the level of COX-2 did not vary much depending on where the tumor was located. The tumor grading was directly proportional to the immunoexpression score. Knowing that high COX-2 levels indicate cancer aggressiveness, this suggests that more aggressive or targeted treatment strategies may be necessary to manage these cases effectively. The ratio of cases with moderate and high immunoexpression (S3 + S4) was higher in younger patients (under 70 years old: 67.16%) compared to those over 71 years old (50%), where low immunoexpression (S1) was more prevalent, suggesting the presence of less aggressive tumors with advancing age [
23,
24].
Cx43 is generally considered to have a tumor-suppressive function. It often has reduced expression or altered function in cancers such as HNSCC, leading to impaired cell communication and uncontrolled cancer cell growth, thus contributing to cancer progression [
14]. Low-expression tumors were most common across all anatomical regions, with extralaryngeal tumors showing especially high rates of low expression, where 95.45% exhibited S1 expression. This may indicate that tumors in the extralaryngeal region have a poorer prognosis in the studied population. In men, 16.25% of the tumors exhibited stronger expression (S2 + S3); whereas in women, all tumors had low expression. According to the literature, this may suggest that women could develop more aggressive head and neck tumors with a poorer prognosis [
25].