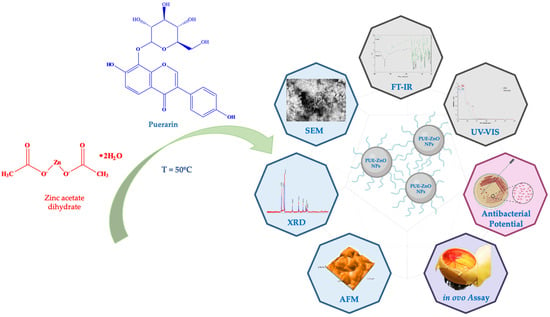
1. Introduction
Nanotechnology and nanoscience research have been leading the way in technological advancements and have experienced significant growth over time [
1,
2]. Nanotechnology provides opportunities for the production of materials, particularly those for medical and pharmaceutical purposes, where conventional approaches may be at their limits [
1].
The term ‘nano’ is typically used to describe particles of matter that are between 10 and 100 nm in diameter, but nanoparticles can be categorized by three size ranges: (i) between 1 and 100 nm; (ii) between 100 and 500 nm; and (iii) larger than 500 nm [
3,
4,
5]. Their unique physical and chemical properties (e.g., surface functionalization, large surface area, tunable porosity) and their increased bioavailability allow them to be utilized as perfect candidates in biomedicine or technology to enhance efficiency and device functionality [
6,
7,
8]. There is a range of nanoparticles available, which include magnetic nanoparticles, carbon nanoparticles, metal-based nanoparticles, lipid-based nanoparticles (e.g., liposomes, niosomes), and polymeric nanoparticles [
6,
7,
9].
Metal-based nanomaterials are used in various fields, including biomedicine, pharmaceutical, cosmetic, biotechnology, catalysis, wastewater treatment, optical sensors, energy storage, and others [
6,
10,
11,
12,
13,
14]. The value of metal-based nanoparticles or metal-based nanostructures is due to their high stability, simple preparation methods, and excellent engineering control over aspects such as size, shape, porosity, surface properties, easy incorporation into hydrophobic and hydrophilic systems, and target cellular penetration capability [
11,
12,
15].
Zinc oxide (ZnO), one of the many varieties of metal oxide nanomaterials, is one of the most widely used metal oxides due to significant features like versatility, nontoxicity, and biocompatibility, which can be customized based on its size, shape, morphology, and surface properties [
15,
16,
17]. Zinc oxide nanoparticles are commonly found in commercial products like sunscreens, ointments, food packaging, and personal care products and have mainly gained popularity in the cosmetic industry as a result of their aesthetic and functional properties, particularly their ability to reflect visible light (e.g., physical sun-blocks or anti-UVA and anti-UVB) [
18,
19]. Therefore, zinc oxide nanoparticles are used in sunscreens and creams to provide effective UV protection [
18]. Cosmetic technology’s preference for a clear appearance is the reason that makes the transparency characteristic of zinc oxide nanoparticles so popular and beneficial for consumers [
19,
20]. Other potential applications of zinc oxide nanoparticles include gene delivery, biosensors, and cancer therapy [
15,
17,
21].
Synthesis of zinc oxide nanoparticles can be achieved using a variety of methods, with the main focus on bottom-up and top-down techniques, using various precursor ZnO salts (e.g., zinc acetate, zinc sulfate, zinc nitrate, zinc chloride) [
22,
23]. Furthermore, numerous studies have demonstrated that zinc oxide nanoparticles can be produced through the use of natural compounds, such as flavonoids (e.g., rutin, curcumin, quercetin, chrysin), which can add biological effects [
24,
25,
26,
27]. The scientific reason behind this is that flavonoids are well known for their affinity for metal ions, and when exposed to them can act as reducing agents, due to the abundance of hydroxyl groups, to generate ZnO nanoparticles [
28,
29,
30]. Moreover, the flavonoids can also be incorporated with nanoparticles to improve their bioavailability and create systems with integrated functions, even though they have a low solubility, poor absorption, and rapid metabolism [
28,
30].
Phytoestrogens, also known as isoflavones, are plant-derived flavonoids that share structural similarities with the hormone estrogen [
31,
32]. The phytochemistry of isoflavones is becoming increasingly important due to their potential for having both estrogenic and antiestrogen effects on the human body [
31,
32,
33]. Puerarin, which is also known as daidzein-8-C-glucoside, an isoflavone found in the roots of the Chinese kudzu plant, possess a variety of biological effects in acute and chronic diseases [
34,
35]. Efforts are underway to overcome the issues of limited bioavailability, low solubility, and low lipid stability in order to utilize puerarin as a therapeutic agent. Researchers have come up with a solution to the mentioned issues by encapsulating different nanoparticles with puerarin, mainly polymeric nanoparticles [
34,
36,
37,
38]. Metallic nanoparticles coated with puerarin were reported as efficient for various biomedical applications. For example, puerarin-coated gold nanoparticles have been successfully used for the detection of ciprofloxacin [
39].
In this context, the purpose of the current study was (i) green synthesis and characterization screening of puerarin-functionalized zinc oxide nanoparticles (abbreviated in our study as PUE-ZnO NPs), without the use of any additional reducing agents; (ii) antimicrobial potential evaluation; and (iii) assessment of in ovo angiogenic and anti-irritative potential (
Figure 1). To our knowledge, there is no other report on the synthesis of zinc oxide nanoparticles using puerarin, physicochemical characterization, antimicrobial potential, and in ovo effects of such nanobiocomposites. Various applications can benefit from the synthesis of ZnO nanoparticles by using puerarin, including pharmaceutical and cosmetic products, cancer therapy, drug delivery, and photocatalytic materials.
2. Materials and Methods
2.1. Chemicals and Microbial Strains
Zinc acetate dihydrate (EP grade) was purchased from CHIMREACTIV SRL (Bucharest, Romania), and puerarin was obtained from BYOSINTH (CAS [3681-99-0], Bratislava, Slovakia). The analysis and quantitative and qualitative measurements involved the following chemicals and materials: dimethyl sulfoxide (DMSO) ≥ 99.5% GC (Sigma Aldrich, St. Louis, MO, USA), five microbial strains (Thermo Scientific, Waltham, MA, USA): Staphylococcus aureus ATCC 25923, Streptococcus pyogenes ATCC 19615, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Candida parapsilosis ATCC 22019, Columbia agar supplemented with 5% sheep blood (Oxoid, Wesel, Germany), Sabouraud agar with chloramphenicol (Oxoid, Wesel, Germany), culture mediums Mueller–Hinton agar and Mueller–Hinton fastidious agar (bioMérieux, Marcy-l’Etoile, France).
2.2. Green Synthesis and Characterization of Zinc Oxide Nanoparticles Using Puerarin (PUE-ZnO NPs)
Briefly, 50 mL of preheated puerarin solution was added into a flask containing 50 mL of zinc acetate dihydrate solution. After 60 min of heating the solution with a magnetic stirrer at 50 °C, it turned milky white, which is a sign of particle formation, and then it uncolored because of particle precipitation. After this, the solution was centrifuged at 10,000 rpm for 10 min, then the supernatant was discarded. The precipitate was collected, washed with distilled water five times to remove any impurities, and dried at room temperature for 24 h (
Figure 2). The resulting dried product (PUE-ZnO NPs) was stored for further analysis.
2.3. Characterization of Green Synthesized PUE-ZnO NPs
The characterization of the PUE-ZnO nanoparticles was performed using ultraviolet-visible (UV-vis) spectroscopy (model: UviLine 9400 Spectrophotometer, SI Analytics, Deutschland, Germany), in a scanning wavelength range of 200–800 nm, to provide a preliminary confirmation of zinc oxide nanoparticles. Fourier transform infrared spectroscopy, FT-IR (model: Bruker Vertex 70 spectrophotometer, Bruker Daltonik GmbH, Bremen, Germany, equipped with a Platinium ATR spectrometer, Bruker Diamond Type A225/Q.I), was performed, and for each sample, 128 scans were recorded in the 4000–400 cm−1 range.
Using X-ray diffraction (XRD), the powder patterns were achieved with a PAnalytical X’Pert MPD diffractometer, using Ni-filtered CuKα radiation (λ = 1.5418 Å), with a scan step of 0.01°, a counting time of 20 s/step, for 2θ ranged between 20° and 80°, and the powdered sample was fine-grounded by hand using an agate mortar and placed in a glass sample holder with a 20 × 20 mm square, 0.5 mm deep recess.
Scanning electron microscopy (SEM) (model: Quanta Feg 250 instrument, FEI, Eindhoven, The Netherlands), which was equipped with energy dispersive X-ray analysis (EDX), was used to identify the surface morphology, size, and elemental composition.
The topography of the PUE-ZnO NPs was investigated by Atomic Force Microscopy (AFM) using a Nanosurf® EasyScan 2 Advanced Research AFM (Liestal, Switzerland). The sample was ground in a mortar with ethanol and then transferred onto a silica glass plate. It was then dried at a temperature of 25 °C. Non-contact mode was used to record the two-dimensional (2D) and three-dimensional (3D) images, with a scanned surface measuring 1.1 μm × 1.1 μm. Nanosurf EasyScan 2 computer software was used to calculate roughness parameters, which included average surface roughness (Sa, nm), maximum peak height (Sp, nm), and maximum valley depth (Sv, nm).
2.4. In Vitro Evaluation of the Antibacterial Activity
Green-synthesized ZnO nanoparticles were evaluated for their antimicrobial activity against five microbial strains (Thermo Scientific, USA). Due to their prevalence in healthcare-associated infections and their ongoing challenges in the biomedical field, the five pathogenic bacterial strains (Gram-negative and Gram-positive bacteria, yeast) were selected and used for testing: Staphylococcus aureus ATCC 25923, Streptococcus pyogenes ATCC 19615, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Candida parapsilosis ATCC 22019.
The antimicrobial activity was evaluated in accordance with the recommendations set forth by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [
40] and the Clinical Laboratory Standard Institute (CLSI) [
41]. Isolation of all bacterial strains was performed on Columbia agar with 5% sheep blood, while Sabouraud agar with chloramphenicol was utilized for
Candida parapsilosis. The concentration of 0.5 McFarland was achieved by preparing the microbial suspensions with 0.85% NaCl.
Determination of the Minimum Inhibitory Concentrations by Dilution Method
A series of dilutions of the tested compounds were performed, with concentrations ranging from 100 to 6.25 mg/mL. In each test tube, 100 µL of each dilution of the PUE-ZnO NPs, 50 µL of Mueller–Hinton agar/Mueller–Hinton fastidious agar and 50 µL of the microbial suspension were added. The minimum inhibitory concentration (MIC) was determined as the lowest concentration exhibited by PUE-ZnO NPs without visible bacteria growth after 24 h at 35 °C [
42,
43].
2.5. In Ovo CAM Assay
The in ovo study was used to assess the potential irritability of the tested samples, as well as to establish if the samples can interfere with the angiogenesis process. For this purpose, the chorioallantoic membrane basic protocol [
44] adapted to our laboratory was implemented. Briefly, the process consisted of incubating fertilized chicken eggs (
Gallus gallus domesticus) at 37 °C in a humidified atmosphere, until the removal of albumin, followed by the opening and subsequent resealing of the upper eggs’ shells.
The irritability test was carried out using the Hen’s Egg Test–Chorioallantoic Membrane Protocol [
45]. As part of the method, the controls utilized are 0.5% sodium dodecyl sulfate (positive control) and distilled water (negative control). During this experiment, test samples in a concentration of 100 μg/mL, next to the positive control and negative control, were placed on the chorioallantoic membrane on day 9 of incubation. The vascular plexus was examined under a stereomicroscope for 300 s to observe vascular change. An irritation score was calculated by analyzing the incidence of vascular events, which enabled us to classify the tested samples into one of the following classifications, according to Luepke [
46]: (i) non-irritant (0 ÷ 0.9); (ii) weak irritant (1 ÷ 4.9); (iii) moderately irritant (5 ÷ 8.9); and (iv) strongly irritant (8.9 ÷ 21). The irritability score was calculated using the following formula (Equation (1)) [
47]:
where the vascular event is measured in seconds and outlined as follows: —hemorrhage event, —lysis event, and —coagulation event.
To assess the changes upon the angiogenesis process, puerarin and PUE-ZnO NPs were prepared with 0.5% DMSO to achieve concentrations of 100 μg/mL, and then 10 μL was placed inside plastic rings located on top of vascularized areas of the chorioallantoic membrane on day 7 of incubation.
The potential vascular changes that occurred due to the tested samples were monitored using stereomicroscopic live analysis and imaging (ZEISS SteREO Discovery.V8, Göttingen, Germany), coupled to a camera (Axiocam 105 color, AxioVision SE64. Rel. 4.9.1 Software, (ZEISS, Göttingen, Germany), while the selected images were processed by ImageJ (
https://imagej.net, accessed on 6 July 2024).