Nanotechnology is the study and application of physicochemical phenomena that manifest and intensify in materials when working at the nanometric scale [
1,
2]. Nanometric materials exhibit enhanced characteristics including increased surface area [
3], and magnetic properties such as superparamagnetism are present [
4]. Nanomaterials also exhibit higher catalytic activity in chemical reactions due to the greater proportion of atoms at the corners and edges compared to the center of the nanoparticle [
5]. This promotes the controlled fabrication of materials in different 2D and 3D nanostructures, leading to improved biosensor capabilities, biodetection, better drug release control [
6,
7,
8], and low-toxicity [
9]. Due to their size, these compounds can be applied in small quantities, making them environmentally friendly and cost-effective [
10]. The transition metals, including Cu and Co, have received significant attention in recent years due to their catalytic, magnetic [
11,
12], optical [
13,
14], and biological properties [
15,
16]. For instance, copper nanoparticles (Cu-NPs) have shown potential industrial applications as gas sensors or in catalytic processes [
17,
18], as well as in high-temperature superconductors and solar cells [
19,
20]. These materials have also been used to replace silver, gold, or platinum nanoparticles in fields such as thermal conductors and microelectronics [
21,
22]. Similarly, cobalt nanoparticles (Co-NPs) have broad applications in areas such as microelectronics, biomedicine, and catalysis [
23,
24]. While Cu-NPs are widely studied due to their cost-effectiveness and availability [
25,
26], Co-NPs have emerged as an alternative, especially in agricultural applications, where they do not induce toxicity [
27]. For example, it has been demonstrated that the use of Co-NPs is effective in aiding the growth of the
Arabidopsis plant, regulating chlorophyll synthesis. Additionally, Co-NPs acted as antibacterial agents in the same plant [
28]. Nanoparticles (NPs) can be synthesized using two techniques: bottom-up and top-down. The bottom-up technique involves the self-assembly of atoms into nuclei to form nanometric materials, using chemical and biological methods. The top-down technique breaks down large materials into small particles through size reduction, employing physical and chemical methods [
29,
30,
31]. Physical methods reduce particle size through grinding, laser pulverization, and evaporation–condensation. Chemical methods include reduction, microemulsion, synthesis in humid environments, spray pyrolysis, precipitation, and microwave-assisted combustion. Furthermore, biological methods, through the use of microorganisms and green synthesis, employ compounds extracted from plants [
31]; these are considered the most economical, and ecological option at present [
32]. The synthesis method influences the final characteristics of the NPs, affecting their size, shape, and stability [
33]. In this regard, the use of electromagnetic radiation has proven to be an important method for obtaining NPs with the desired size and shape [
34,
35]. It has been shown that by carefully adjusting the electromagnetic source, both the size and shape of the NPs can be controlled [
36]. These characteristics are important for their effectiveness in various applications, including plant disease control, where they can induce oxidative stress, modifying cell membranes and organelles, and inhibiting fungal growth and spore germination [
37].
Green synthesis or biosynthesis of NPs uses active principles from plants, fungi, or bacteria, offering environmental and economic advantages over physical and chemical methods [
42]. This bottom-up process involves reduction/oxidation reactions, where microbial enzymes or plant phytochemicals reduce metal compounds to NPs [
43]. Shedbalkar et al. point out that biosynthetic NPs are more stable, morphologically controllable, and scalable [
44]. Tesfaye et al. mention that active components such as flavonoids, saponins, alkaloids, tannins, and plant phenolics can reduce metal salts into stable NPs [
45]. Studies have demonstrated successful synthesis of NPs using various plant extracts, reaching controlled morphology and enhanced stability [
46,
47]. Research highlights include the use of
Mentha pulegium,
Eucalyptus globulus, and
M. calabura for synthesis, as well as ascorbic acid as a reducer, showcasing applications in plant disease control and growth enhancement [
48,
49]. In particular,
Medicago sativa (alfalfa) presents an excellent candidate for green synthesis due to its rich content of bioactive secondary metabolites, including terpenoids, polyphenols, and flavonoids [
50,
51]. The integration of green principles with hydrothermal methodology creates a sustainable approach that combines the morphological control of hydrothermal synthesis with the eco-friendly characteristics of green synthesis [
52,
53,
54].
On the other hand, the incorporation of NPs into biopolymer matrices is an important strategy to improve their stability and application [
55]. Natural biopolymers such as chitosan (CS) are used to achieve this objective. CS, a natural biopolymer derived from chitin, has garnered significant interest due to its biocompatibility, biodegradability, and ability to form stable matrices with metal-NPs [
56]. In this context, composite characterization is necessary to obtain information about the distribution of NPs within the matrix and the nature of polymer–metal interactions. A promising application of these NPs is their use in the agricultural area, where there are challenges, such as the biological control of black Sigatoka disease, caused by the fungus
Pseudocercospora fijiensis, which can reduce banana crop yields by up to 80% [
57,
58]. These crops are extremely important for Ecuador, a country that leads the export of this fruit and benefits 2.5 million people by generating income and employment [
59]. While traditional chemical control methods remain prevalent, environmental concerns and pathogen resistance have driven research toward alternative solutions [
60], with characterized and stabilized NPs that offer a reliable and effective long-term strategy [
61].
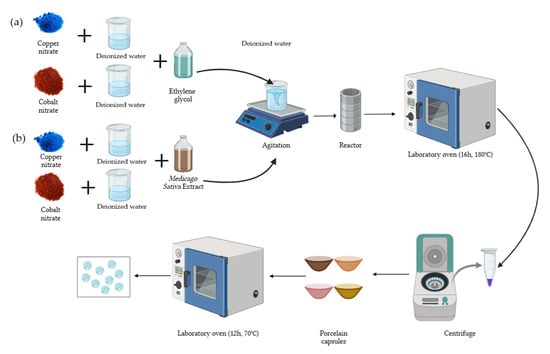
This research aims to conduct a comparative study of Cu-NPs and Co-NPs synthesized through solvothermal and green hydrothermal synthesis methods using alfalfa extract. The investigation focuses on comprehensive physicochemical characterization using UV–vis, AFM, SEM, and FTIR analysis to understand how different synthesis routes influence NP properties and their antifungal potential against Pseudocercospora fijiensis. SEM was employed to characterize the nanocomposite structure and confirm the successful incorporation of metal NPs. This comparative approach will provide valuable insights into the relationships between synthesis methods, NPs characteristics, and their potential agricultural applications.
Source link
Tania Caguana www.mdpi.com