1. Introduction
Drought stress has become a major destructive factor among all abiotic stresses, which has led to an unfavorable decrease of 45% in the development of agricultural crop production throughout the world [
1]. Additionally, drought stress worsens the quality of grain and crop yields as a consequence of alterations in plant physiological functions [
2]; furthermore, drought stress leads to biochemical modifications and physical damage at the overall organism and cellular levels [
3]. Drought also has the capability to block photosynthesis, respiration, and stomatal movement, which are further constraints in plant growth and physiological metabolism [
4]. Drought resistance is a wide term that includes certain adaptive mechanisms or features, such as drought escape, drought tolerance, and drought avoidance, that plants use to manage the effects of drought stress [
5,
6]. An increase in the abscisic acid concentration is often noticed under drought and other abiotic stresses [
7]. When a crop is sensitive to drought stress, it leads to the following effects: declined levels of photosynthesis [
8] and other processes, a smaller leaf area index [
9], stomatal closure, a reduction in CO
2 intake, an increase in photo-respiration, limited carboxylation, decreased water potential, a reduction in Rubisco activity, an increase in reactive oxygen species (ROS) [
10], oxidative damage to chloroplasts and plasma membranes, the downregulation of non-cyclic transport, obstruction in ATP synthesis, a reduction in nutrient uptake, susceptibility towards disease, and decreased yield quality and production [
11]. There are different types of ROS that are produced in plants, namely H
2O
2 (hydrogen peroxide), O
2−1 (singlet oxygen), CO (carbon mono-oxide), NO
x (nitroxide), SO
2 (sulfur dioxide) [
12], O
2•– (superoxide anions), and OH (hydroxyl radicals) [
10,
13].
The production of ROS in plants acts as an alarm signal that could further kickstart the defense mechanism or acclimatization process [
14]. Sodium dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), citrullines, polyamines, and several enzymes act as antioxidants, which minimizes the effects of drought stress [
15]. Ascorbic acid (AsA) and glutathione are the low-molecular-weight antioxidants that are present in nearly all organelles, and they are associated with ROS quenching, initially by lending reducing equivalents to ROS-scavenging enzymes [
13,
16]. Thus, this process has the capability to maintain the decreased levels of ROS concentrations even if ROS are produced at a high rate as these enzymes can directly react with ROS [
17,
18]. Plant growth hormones such as auxins, cytokinins, gibberellins, abscisic acid, and salicylic acid alter plant responses to drought stress [
15]. Defense hormones such as jasmonic acid (known as a first defense hormone) and its derivatives, such as methyl ester (MeJA) and isoleucine conjugate (JA-Ile), are known together as jasmonates. They play a major regulatory role in different aspects of plant growth and development [
19]. JA, JA-Ile, cis-OPDA, ABA, and SA have the ability to improve stress tolerance in plants; along with this, SA helps plants in abiotic stress mitigation [
20]. ABA is an essential mediator of drought stress, and it has the capability to regulate plant water balance and provide osmotic stress tolerance [
21]. OPDA (precursor of JAs) has a vital role in drought tolerance through various signaling pathways, and it remains different from JA-Ile [
22].
The circadian clock in plants is often known to be a central oscillator or internal biological timekeeper that helps plants receive environmental cues such as light, humidity, nutrients [
23], and temperature (the input pathway has the capability to reset the clock) [
24,
25], and it supports roughly 24 h of oscillation (16 h light and 8 h dark) in the expression of genes, which manages the changes with output genes [
26]. Moreover, the circadian clock helps in managing photo-periodic rhythmicity for the enhanced growth, development, and fitness of plants [
27]. Over the last few decades, it has been revealed that the circadian oscillator in plants has the ability to provide them stability towards the adaptation or alleviation of stress [
28]. The clock consisting of a self-sustained mechanism and its various transcriptional and translational feedback loops (TTFLs) are well maintained in plant species [
27,
29]. This biological clock has a control on a set of physiological and biochemical pathways likely seed germination, photosynthesis, hypocotyl elongation, flowering, stomatal movement, respiration, ion uptake, sugar metabolism, nutrient metabolism, translocation, phytohormones, and senescence [
14,
30]. The clock genes regulate each other’s expression, having certain positive and negative feedback loops; also, these genes are expressed especially at a particular time period of the day [
24]. The photo-periodic rhythmicity of the biological clock starts with an input pathway where the external environmental cues, such as light (daily dawn and dusk =
zeitgeber) [
31] and temperature, pass the light signals [
32] to photo-receptors, namely phytochromes (PHYs) and cryptochromes (CRYs) [
33,
34]. Then, these light stimuli are transported (light-signal transduction) and integrated into the clock [
35].
Spinach (
Spinacia oleracea) is a green leafy plant (economically crucial crop) and has its native origin in the central and southwestern regions of Asia [
36,
37]. It belongs to the order Caryophyllales, the family Amaranthaceae, and subfamily Chenopodioideae [
38,
39,
40]. The leading producers of
Spinacia oleracea L. in India are Telangana, Kerala, Tamil Nadu, Karnataka, Maharashtra, West Bengal, Andhra Pradesh, and Gujarat [
41]. The optimum range of temperature for growing spinach is between 15–30 °C, along with a rainfall of about 80 to 120 cm; usually, they grow up to the height of 30 cm (along with a stem of 50–90 cm high [
42]) and are nearly 15 cm wide during harvesting stage [
41]. Spinach is a well-known long-day plant; the arrangement of
S. oleracea appears to be alternate, and the true leaves are formed during days 4–11 after seedlings [
42]. A rapid and sudden change in climate, along with reduced availability of water, restricts spinach cultivation [
43]; the production of cultivated spinach per annum is 30 million tons worldwide [
44]. Spinach leaves have a rich source of vitamins of the B group, ascorbic acid, micro-nutrients, phytonutrients, and beta-carotene (or pro-vitamin A) [
40,
41]. The seeds of spinach genotypes are of various forms like round, pointed, or prickly, and the texture of the leaf is flat, smooth, semi-savoy, or crinkled (=savoy) [
39]. Also, there are early and late genotypes that may vary promptly during stem elongation according to the leaf rosette [
40]. It has a shallow root architecture, and it needs regular irrigation to maintain the soil moisture content for constant leaf growth and development [
45].
The current research was orchestrated with the aim to explore the effect of drought stress on horticultural crop spinach (Spinacia oleracea L.) genotypes (Delhi Green (DG) and Malav Jyoti (MJ)) on the basis of 12 h of photo-periodic rhythmicity at the time intervals of 4 h; this is exactly by 6 and 10 a.m. and 2 and 6 p.m. on day 5 (stressed period) and day 10 (drought re-irrigated period). The focus of this research was to furnish an overview of the investigation of drought-resistant and drought-sensitive spinach genotypes with specific measurements such as morpho-physiological and photosynthetic variables, oxidative parameters, cytotoxic analysis, phytochemicals (phenol), protein, and thylakoidal proteome analysis. Moreover, there are no preceding studies on the impact of drought stress on spinach cultivars at the cellular level or ROS-scavenging mechanism, nor are they accompanied by data of molecular-level analyses of phytohormones concentrations along with circadian and drought gene-regulatory networks (having CCA1, LHY, TOC1, RVE8, PRR3, PRR5, PRR7, PRR9; and DREB1, DREB2, PIP1) in spinach genotypes on day 5 and day 10 (10 a.m. and 2 p.m.). From our findings, we hypothesized that the circadian core oscillators play a vital role, especially in alleviating drought stress by altering the physiological parameters such as photosynthesis, antioxidative mechanisms, chloroplast proteome, and the gene-regulatory network along with targeted metabolomics, mainly during particular periods (10 a.m. and 2 p.m.) of the circadian cycle.
4. Discussion
Drought is one of the major factors that has a limitation on spinach growth and productivity in water-circumscribed environments [
70]. It has been observed that the plant circadian clock is associated with the alleviation of abiotic stress [
71]. There are few reviews (or a restricted number of research works) that have reported on how the circadian clock is associated with stress tolerance. The circadian oscillator is closely related to the adaptability of plants as it is accompanied by physiological processes depending upon the changes in environmental cues [
72,
73]. The circadian clock plays an essential role in acclimatizing the external environmental stresses, mainly drought stress [
74]. Consequently, the current study has mainly focused on exploring three major factors: (i) How the spinach plants (MJ and DG) tolerate or combat drought stress by employing the internal circadian oscillator, provided with molecular-level analysis, including chloroplast proteome, particularly during the morning–evening loop. (ii) The current study has concentrated on providing knowledge about how the circadian clock gene and drought-responsive genes are regulated during photo-periodic hours (10 a.m. and 2 p.m.) on day 5 and day 10. (iii) We have also conducted a study on targeted metabolomics analysis with phytohormone concentrations in spinach genotypes, especially defense hormones such as ABA, JA, SA, JA-Ile, and cis-OPDA under both water regimes (drought stress and after re-irrigation).
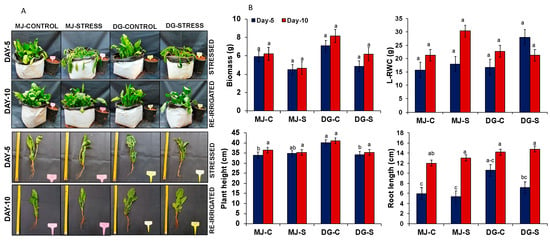
The apparent symptoms of drought stress during the vegetative stage are a reduction in plant height, wilting of leaves, and changes in the number and area of the leaves [
4]. The leaves of plants acquire smaller leaf areas, higher leaf tissue density, and larger leaf thicknesses to reconcile drought conditions [
75]. The roots of the plants also play a vital role in drought stress [
76]; here, spinach mainly uptakes the macro-nutrients present in the soil, helping the plants survive by absorbing and utilizing the water stored in the soil [
77]. The plant height is grievously affected by drought, and it is correlated to leaf senescence and cell enlargement [
4]. Similar to our results, there was a significant reduction in plant height noticed in maize hybrids [
78], lily [
79], rice [
80], and sugarcane [
81] under drought stress. The decreased plant height and biomass were observed in durum wheat under drought conditions [
82]; these results were noticed to be similar to our results, which are presented in
Figure 1A,B. The reduction in plant height is particularly due to a reduction in cell expansion, impaired mitosis, and a rise in leaf shedding under drought stress. Aside from the plant height, leaves are major organs for transpiration and assimilation in plants [
4]. In our study, there was an increase in RWC, mostly resulting in high osmotic regulation or lower elasticity of the tissue cell wall [
83]. Similar to our results (
Figure 1A,B), Zokaee-Khosroshahi [
84] has identified a significant decrease in growth parameters, namely the dry and fresh weights, number of leaves, and total leaf area by using five Iranian almond species (
Prunus dulcis,
P. haussknechti,
P. eburnean,
P. scoparia, and
P. eleagnifolia) under polyethylene glycol (PEG)-induced drought stress. In a recent study, there was a reduction observed in plant height, root length, and the dry and fresh weights of two canola cultivars when it was treated with 300 g/L of PEG drought stress [
85].
Photosynthesis is one of the essential processes that is affected by drought stress. Leaf photosynthetic products are known to be a foundation material for plant growth. The reason behind the reduction in NPR and transpiration rate is the decline in soil RWC. Plant photosynthesis and yield are directly affected due to the changes occurring in leaf area, which is nearly an obvious feature that can be observed from plant leaves under drought stress [
4]. The photosynthetic rate decreases due to the closure of the stomata, disturbances in certain enzymatic activity (especially those involved in ATP synthesis), and membrane damage [
15]. In our study, we noticed a reduction in the photosynthetic parameters under drought stress (
Figure 2); however, during day 10 after re-irrigation, 10 a.m. and 2 p.m. (
Figure 2) showed an increase in photosynthetic parameters, which indicates that these photo-periodic hours in circadian biology have helped the spinach genotypes in combating the effect of drought stress. The prime reason behind the decline in NPR under drought stress is stomatal limitation and non-stomatal limitation. Under mild drought, stomatal limitation is the major reason, whereas during severe drought conditions, non-stomatal factors are the principal reason behind the decrease in NPR [
86].
Thus, chlorophyll is subsequently metabolized in plants and is correlated to photosynthesis. The decreased chlorophyll pigments (green pigments) under drought stress cause differences in photosynthetic function [
87]. Our results (
Figure 3A) are partially similar (we observed a reduction in carotenoid content under drought stress) to Wu et al. [
88], where it was found that the total chlorophyll content and carotenoids for Chinese cork oak (
Quercus variabilis) seedlings have shown a significant reduction under various drought intensities (40% and 20%). However, it has been observed that not all plants show a decreased chlorophyll content, and also, has pointed out the increase in chlorophyll content in borage leaves under drought stress, which was stated mainly due to a lower leaf area index and high radiation interception [
89]. We have also observed an increased chlorophyll content in our results, presented in
Figure 3A. The alteration in chlorophyll pigments often leads to a change in the color of the plant to yellowish-brown under drought-stress conditions [
4]. Also, our study focused on chloroplast proteome to understand the effect of drought stress in spinach genotypes on day 5 (10 a.m. and 2 p.m.), presented in
Figure 3B; alongside this, our study has shown how exactly the circadian pattern controls the harmful effects of drought stress with the re-irrigated spinach samples on day 10 (10 a.m. and 2 p.m.).
Due to the induction of drought stress, plants undergo a set of secondary stressors, such as osmotic and oxidative stress. As MDA and proline are known to be stress indicators, this current study has initially focused on knowing how the circadian oscillator helps spinach combat drought stress. Similar to our results with certain samples, there was a decrease in MDA and proline contents observed in leucaena seedlings under drought stress [
90]. In this study, we observed an increased MDA content (
Figure 4A) with most of the stressed samples, as drought stress leads to an increase in MDA content, which is a LPO (lipid peroxidation) indicator; due to the increase in LPO, the plant undergoes membrane damage, which results in a reduction in growth, which shows an increase in MDA (bi-product of LPO) content. Plants have the ability to withstand dehydration (plants do this by involving their defense systems to reciprocate oxidative stress) using various physiological activities, namely osmotic adjustment through osmoprotectants (or osmolytes) such as amino acids (betaine and proline), sugars, polyols, organic, inorganic ions, and other amino acids [
91,
92]. These osmolytes play an important role in maintaining the plant’s cellular functions under drought stress [
15,
87]. The osmolyte or osmotic regulating substance proline is also known as a type of free radical scavenger [
93], which is readily stored in the vacuoles of plants [
94]. In this study, we have observed an increase in proline content (
Figure 4B) with an increase in proline accumulation, as proline has a vital role in reducing the deleterious effects of drought and helps the plants with their stress tolerance by safeguarding enzyme protein structures and membranes [
95].
In an extreme stress condition, it leads to an increase in ROS concentrations in cells and causes oxidative damage to membranes (LPO), proteins, DNA, and RNA molecules. Thus, it further leads to oxidative destruction at a cellular level, and it is known to be oxidative stress [
96]. ROS metabolism is foremost important with respect to the maintenance of the oxidative physiology of plants [
10]. In green plants, chloroplasts are the major site for the production of ROS [
97]. The production and accumulation of ROS in spinach genotypes have been observed in our study, and it is presented in
Figure 4C,D. In a previous study, it has been reported that drought stress has the ability to increase ROS, along with a severe or partial rise in oxidation of cellular level components in plants [
10]. ROS (acts as signaling molecules) is advantageous to plants under drought stress and allows them to regulate their metabolism; it also supports the acclimation process in plants during abiotic stress [
98]. Nevertheless, this process can be mitigated in cells through a set of ROS-detoxifying proteins and antioxidant enzymes such as SOD, CAT, and APX [
99]. Our results have shown a significant variance during the morning–afternoon loop (10 a.m. and 2 p.m.) on both day 5 and day 10 (
Figure 6A). TPC has significantly elevated in spinach genotypes (
Figure 5B) under drought stress; these are compounds that are naturally present in plants and are produced in the cytoplasm and endoplasmic reticulum. This plays a beneficial role as a signal molecule, scavenges the excess ROS, and also acts as a secondary antioxidant defense system under stressed conditions [
95]. TPC was observed to be high in concentrations in various safflower cultivars under water stress [
100]. The accumulation of TSP in leaf samples of spinach genotypes under drought stress acts as an osmotic adjustment in plants. In the present investigation, TSP in spinach leaves was not affected significantly under drought stress (
Figure 5A).
With the aid of the results obtained from the photosynthetic parameter, stress indicators, photosynthetic pigments, and antioxidant activities—which have shown a significant variance between 10 a.m. and 2 p.m. (morning-afternoon loop) on both day 5 and day 10—were selected for conducting isozymes (as a proof for scavenging enzymes), protein analysis, gene expression studies, and metabolite concentration analyses. The different isoforms of antioxidant enzymes were separated using the native gel technique using an electric field where H
2O
2 serves as the substrate compound. In the current study, we observed the three different isoforms of SOD isozymes, which may be Mn SOD, Cu/Zn, and SOD (
Figure 6B). The three different isoforms of APX may include thylakoidal APX (tAPX), stromal APX (sAPX), and cytosol APX (cAPX). The isoforms of CAT may include iron porphyrin enzymes. The chloroplast is known to be the site of photosynthesis in the leaves of green plants, and chlorophyll is utilized by chloroplast for absorbing, transferring, and transforming light energy [
4]. To evaluate the effect of drought stress in spinach genotypes, a molecular-level analysis was conducted using an essential tool, chloroplast proteomics. There was a study reported previously in Okra [
48] with changes in thylakoidal proteome under drought stress, but this was neither on the basis of circadian hours nor after a re-irrigated regime. We observed a downregulated expression of thylakoidal proteins with drought-stress samples (
Figure 3B) and have shown an upregulation in the expression of these photosynthetic proteins with respect to photo-periodic hours. These results have supported the results of the biochemical processes performed for the photosynthetic parameters and photosynthetic pigments.
The relative expression of the core genes of a circadian oscillator, such as
CCA1,
LHY,
TOC1, and
RVE8 in spinach genotypes (MJ and DG) between 10 a.m. and 2 p.m. on day 5 (drought samples) and day 10 (re-irrigated samples) has been elucidated; and it is presented in
Figure 7. The morning-phased circadian clock is expressed highly in the mesophyll tissues, whereas the evening-phased circadian clock is increasingly expressed mainly in vascular systems [
101]. The two single clock-regulated Myb-like transcription factors (TFs), such as
circadian clock associated 1 (
CCA1) and
late elongated hypocotyl (
LHY), are the core members of the circadian clock, which becomes expressed in the dawn period of the day [
101,
102,
103].
CCA1 and
LHY are the morning-expressed TFs but are inhibited during the afternoon/evening by the expression of
PRRs, mainly
TOC1 [
104];
CCA1 and
TOC1 often form an auto-regulatory positive or negative feedback loop [
105].
TOC1 (one of the small subfamilies of PRR proteins) is an evening-expressed gene that becomes activated by CCA1 or LHY [
106].
CCA1,
LHY, and PRRs (1, 3, 5, 7, 9) are expressed during the morning-to-evening loop, and also, they consist of core negative feedback loops [
107,
108]. The continuously expressed (morning to midnight)
PRRs (1, 3, 5, 7, 9) inhibit the transcript accumulation of
CCA1,
LHY, and
RVE8 [
101]. From a recent report, it was known that
CCA1,
LHY, and
RVE8 occur during dawn and reach the highest increase by the morning time period [
25]. The
Reveille (
RVE) member
RVE8 is a homolog of
CCA1 and
LHY, which is an essential TF that activates the evening genes by binding to the promoter regions of the evening element (EE) motif [
109,
110]; also, it is expressed during the afternoon time and has the target of expression similar to
CCA1 and
LHY [
110,
111].
RVE8 (Myb-like TF) is a homolog of
CCA1 and
LHY, which becomes activated and expressed during the mid-day or afternoon hours of the circadian system [
24,
109].
The PRRs are a family of genes that are known to be an integral part of the circadian clock; they have a vital role in combination with the input pathway, central oscillator, and output pathway [
112]. As the role of PRRs is not fully understood yet in green plants, this current study has a major focus on PRRs in spinach genotypes (MJ and DG). The relative expressions of
PRR3,
PRR5,
PRR7, and
PRR9 have been observed in spinach genotypes between 10 a.m. and 2 p.m. on day 5 and day 10 (under drought stress and after re-irrigation), and it is presented on
Figure 8. The PRR genes have the ability to alter the phenotypes of output processes in the circadian clock, such as hypocotyl elongation, drought-stress responses, and flowering time, which indicates their importance towards biological processes [
113]. These PRRs act as transcriptional repressors towards morning-phased circadian clock genes [
114,
115]; also, PRRs are known as a regulatory (gating) mechanism that alters plant responses and regulates plant growth [
112,
116]. The
PRR3 gene provides stability for the TOC1 protein by arresting the interactions between F-box protein ZEITLUPE (ZTL) and
TOC1; thus, the expressions of
TOC1 and
PRR3 may remain in a similar pattern.
PRR3 is a gene that is often expressed in the vasculature of cotyledons and leaves; also, it is co-regulated with
TOC1 [
117].
RVE8 binds to the promotor regions (consists of Evening Element motifs) of both
TOC1 and
PRR5;
RVE8 and
PRR5 form a negative feedback loop [
109].
CCA1/LHY inhibits the expressions of
PRR3,
TOC1, and
PRR5, whereas it indirectly activates the transcription of
PRR7 and
PRR9 (102). The
CCA1 and
LHY TFs directly repress the evening genes and play a crucial role in activating
PRR7 and
PRR9 [
118,
119], whereas
CCA1/
LHY are repressed by the
PRR genes directly from afternoon until midnight [
114,
119]. The small subfamily of proteins, such as
pseudo-response regulators (
PRR7 and
PRR9), are morning-phased genes, whereas
PRR5,
PRR3, and
PRR1 (or
TOC1 (
Timing of CAB expression 1)) are evening-phased genes [
120,
121]. In spinach genotypes (MJ and DG), the core and
PRR genes have been shown as either upregulated or downregulated expression patterns (even during their respective photo-periodic hour) on day 5 due to the effect of drought stress on plants (
Figure 7 and
Figure 8). However, by day 10, after re-watering, these genes depict their expression according to their photo-periodic hours with the help of the circadian clock.
We observed upregulation in the expression levels of
DREB1,
DREB2, and
PIP1 under drought stress, whereas it has shown downregulation in expression patterns after re-irrigation with respect to the circadian hours (
Figure 9). The
DREB genes have shown an increased expression under drought stress in previous studies performed in Grape (
Vitis vinifera L.) [
122] and bread wheat cultivars [
123]. Dehydrative responsive element binding (DREB) acts as a crucial TF [
124] from the ethylene responsive factor (ERF) family (126) that can manage the expression of various stress-inducible genes [
123]. There are two sub-classes of DREB, such as
DREB1/CBF and
DREB2;
DREB1 and
DREB2 can remarkably increase plant tolerance towards abiotic stresses, mainly water stress [
125,
126]. Plasma membrane intrinsic proteins (PIPs) are a subfamily of aquaporins (AQPs-drought inducible protein) [
127] and drought-responsive genes, which have a main role in water permeability [
128]; moreover, it has been previously reported that, in Arabidopsis, isoforms of
PIP1 have low or zero water channel activity, comparatively [
128]. The upregulated expressions of
PIP genes were observed in
Phaseolus vulgaris plants due to the direct effect of low water content and reduction in soil water potential. It was also proposed that aquaporins have the capability to serve as osmo-sensors in plant membranes [
129]. The reduction after re-watering, along with the photo-periodic hours, may indicate the role of circadian biology in controlling the effect of drought stress in spinach genotypes on day 10. Apart from the gene-regulatory network, there are several other factors that can play a vital role in maintaining defense mechanisms in plants. One of the important factors is metabolomes, in particular, with stress phytohormones that play a crucial role in maintaining drought stress, combining with gene interplays.
An intention of the analysis of targeted metabolomics by UHPLC-MS/MS was to estimate the amount of each defense hormone present in leaf samples of spinach genotypes with respect to circadian biology. With the help of this result, we were able to elucidate the concentrations of each metabolite present in each sample during the morning–afternoon loop. The defense hormones, such as JA, ABA, and SA, function as chemical signals or messengers towards environmental stresses; the stress signals provoke the initiation of a set of plant developmental and physiological processes, including stomatal closure, osmolyte accumulation, and stimulation of root growth. This further leads to a reduction in water loss and acclimation to the stressed conditions [
130]. We observed an increase in the concentration of ABA in spinach genotypes under drought stress during both photo-periodic hours on day 5 (
Figure 10). Under drought-stressed conditions, ABA is highly produced and accumulated in guard cells that are present around stomata; and it promotes stomatal closure, which prevents water loss and leads to water conservation [
131]. The reduction of ABA after re-irrigation on day 10 on the basis of circadian biology has shown the recovery of spinach genotypes from drought stress. Plant hormones are an essential factor that helps plants withstand unfavorable conditions like drought stress [
132]. SA is an important cellular regulator in plants that serves as a signaling molecule for activating the defense mechanism [
133]. It has been reported in
Arabidopsis thaliana that the accumulation of JA in leaf samples was essential for a significant rise in JA-Ile. The increase in JA and JA-Ile is associated with an increase in cis-OPDA [
134]. This indicates that JA, JA-Ile, and cis-OPDA are directly related to each other and may remain in the same pattern while being quantified, which is similar to our results that have been presented in
Figure 10. These defense hormones have the ability to increase the antioxidant enzyme systems (enzymatic and non-enzymatic antioxidants), decrease membrane damage, and improve plant growth and biomass production. The quantification of ABA, JA, SA, JA-Ile, and OPDA has been reported in
Arabidopsis thaliana and
Citrus sinensis by LC-MS/MS in a previous study [
135]. Thus, the evaluation of these metabolite concentrations in leaves of spinach genotypes (MJ and DG) during both water regimes (drought stress and re-irrigated condition) revealed that the overall recovery of phytohormones present in spinach genotypes from drought stress and mainly after re-irrigation is on the basis of photo-periodic hours. The overall schematic overview of ROS mitigation caused by drought stress is described in
Figure 11.