1. Introduction
Campylobacter sp. and
Salmonella sp. are zoonotic foodborne agents and two of the most common infectious causes of gastroenteritis worldwide [
1,
2]. These infections represent a significant impact on global morbidity and economic burden. Infection by these agents is usually benign, presenting as acute gastroenteritis [
2], but both have been implicated in gastrointestinal acute complications (such as toxic megacolon, perforation, and hemorrhage—[
3,
4,
5,
6,
7]) and invasive disease, with bacteremia and extraintestinal metastatic infections, including meningitis, bone and joint infections, endovascular infections, and endocarditis [
8]. In this setting, mortality can be as high as 15% [
9]. Additionally, infection by
Campylobacter sp. has been associated with autoimmune and inflammatory conditions such as Guillain–Barré syndrome (GBS), inflammatory intestinal disease, irritable bowel syndrome and celiac disease [
1]. Reactive arthritis is present in 3–13% of
C. jejuni-infected patients [
10] and in 0.07% of GBS cases [
11], making this species the most commonly recognized infectious cause of the syndrome, being responsible for 5–41% of cases [
12]. Invasive infections by non-typhoidal
Salmonella sp. (NTS) and
Campylobacter sp. in the European setting have mostly been linked to old age and immunosuppression [
13,
14]. For NTS, some of the most extensively studied types of immunosuppression include solid and hematologic malignancies [
15] and chronic immunosuppressive therapy, especially anti-TNF-α drugs [
16]. HIV infection/AIDS has been linked with recurrent invasive NTS infection [
17]. Hypogammaglobulinemia, either primary or secondary, seems to be a risk factor for invasive and recurrent campylobacteriosis [
18].
In the European region, campylobacteriosis and salmonellosis are the two most commonly reported gastrointestinal infections and important causes of foodborne disease outbreaks. Their hospitalization rate is significant (21.0–29.9%), but mortality is considerably low (0.05–0.19%). At a global level, the more frequently identified
Campylobacter species are
C. jejuni (88.1%),
C. coli (10.6%),
C. fetus (0.16%),
C. lari (0.11%), and
C. upsaliensis (0.09%) [
19]. Three
Salmonella enterica subsp.
enterica serovars accounted for 70.3% of human cases in 2019:
S. enteritidis (48.7%),
S. typhimurium (12.4%), and monophasic
S. typhimurium 1,4,[5],12:i:- (11.1%) [
19]. Resistance to quinolones is common in Europe both in
Campylobacter (60–70%) and
Salmonella (around 15%) [
20].
However, marked differences are reported between European countries. In Portugal, systematic declaration of cases of salmonellosis was implemented before 2010, but declaration of campylobacteriosis cases to the National Epidemiological Surveillance System (SINAVE, from the Portuguese Sistema Nacional de Vigilância Epidemiológica) only started in 2015. Estimates of the incidence of
Campylobacter sp. infection and clinical descriptions of cases before this period are scarce and dispersed, with only a few studies being published during that time. These studies [
21,
22,
23,
24,
25,
26] showed positivity rates from diarrhea samples of 5.3–31.9% for
Campylobacter sp. and 14.8–19.4% for
Salmonella sp., both predominantly isolated in children under 5 years old. Rates of isolation showed an increasing trend for
Campylobacter sp. but a stability for
Salmonella sp.
C. jejuni was the most commonly identified species, followed by
C. coli or C.
concisus, depending on the studies. Resistance to quinolones in
Campylobacter sp. was generally >50% (and >90% in the most recent studies) and resistance to macrolides was 6.5–20%; rising trends of resistance for both antibiotic classes were noted. Most of these studies comprised a small temporal scale and identified fewer than 150 patients with
Campylobacter sp. or non-typhoidal
Salmonella sp. infection. Studies of a larger magnitude have been conducted in other countries and are needed in Portugal [
27].
In Portugal, systematic surveillance after 2015 and additional hospital-based studies have been suggesting similar findings: macrolide resistance in
Campylobacter sp. exceeds 10% [
28]; combined resistance to macrolides and fluoroquinoles in human isolates is one of the highest in European countries, especially in
C. coli (36.8%) [
20]; and incidence rates show an increasing trend for
Campylobacter sp. and stable for
Salmonella sp. [
19]. Even so, incidence rates estimated by the number of cases officially reported were much lower than the average in the EU (7.7 vs. 40.3 confirmed cases/100,000 population for
Campylobacter sp. and 2.5 vs. 13.7 for
Salmonella sp.) and the incidences calculated for neighboring countries [
19]. On the other hand, Portugal reported one of the highest hospitalization rates in 2017 (84%). These differences likely reflect distinct reporting practices, leading to a greater underreporting of mild disease and a primary focus on severe disease. This hypothesis has been supported by studies on gastroenteritis in returned travelers [
29] and modeling studies [
30] that suggest only 1/2080 to 1/93 cases of salmonellosis are reported to the national monitoring system, putting Portugal among the European countries with the highest true incidences and simultaneously making it one of the countries with highest underreporting rates.
The objectives of this study are to describe the epidemiology, clinical presentation, and management of Campylobacter sp. and Salmonella sp. infection in a tertiary hospital in Northern Portugal between 2010 and 2020 and to determine reporting frequency and identify factors associated with underreporting of cases. This study also aims to investigate risk factors for invasive disease and hospitalization, and to compare these outcomes according to Campylobacter sp./Salmonella sp. species/serovar.
2. Materials and Methods
2.1. Study Population and Elegibility Criteria
A single-center retrospective study was conducted in São João University Hospital, a tertiary public healthcare provider in the Oporto metropolitan area, in Northern Portugal (a country located in Southwest Europe, bordering Spain and the Atlantic Ocean). Patients diagnosed with Campylobacter sp. or Salmonella sp. infections were included in the study. The period of analysis for this study was 2010–2020 for Salmonella sp. and 2015–2020 for Campylobacter sp., inclusively.
2.2. Microbiological Diagnosis
Patients were retrospectively identified by the Clinical Pathology department via a database search. Positive microbiological culture results were screened automatically for the keywords “Salmonella” and “Campylobacter”. Regarding the cultural exam protocol, since 2016, stool samples have been systematically sent to the laboratory in a transport medium (ETM® Enteric Transport System—Alpha-Tec). Selective culture media were used for Campylobacter sp. and Salmonella sp. Since 2019, incubation at 42 °C with a microaerophilic atmosphere has been implemented for Campylobacter sp. isolation. Identification of Salmonella sp. was performed using VITEK® 2 GN (bioMérieux Inc., Durham, NC, United States of America [USA]) identification cards and identification of Campylobacter sp. was performed by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (using VITEK® MS). Antimicrobial susceptibility testing was performed using the automated VITEK® 2 System for Salmonella sp. and by disk diffusion for Campylobacter sp., with interpretation according to the most updated clinical breakpoints of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) at the time. Serotyping of Salmonella enterica isolates was performed by sera agglutination tests using BIORAD® (Hercules, CA, USA) kits. A list of serological testing performed in the Hospital was similarly obtained and the positive results were selected for analysis. The search for antibodies against Salmonella Typhi or Paratyphi was performed using the Widal indirect agglutination test and a positive result was admitted when antibody titers against O or H antigen increased by at least four-fold between serum samples taken two or more weeks apart. Finally, a list of samples where Campylobacter sp. DNA was identified by real-time polymerase chain reaction (PCR) was retrieved. Commercial Campylobacter PCR Detection kits (Bio-Rad®) were used. All types of samples were included in this search, regardless of the department where they were collected. Patients with one or more positive results (culture, serology, and/or PCR) were considered infected by Salmonella sp. and/or Campylobacter sp. and were included in the study.
2.3. Data Collection, Variable Definitions, and Grouping
Data from the patients were extracted directly from the medical records respective to the episodes when Campylobacter sp./Salmonella sp. infection was diagnosed through a standardized procedure. Sociodemographic and clinical information was retrieved for each patient, including clinical presentation (signs/symptoms), underlying conditions, diagnosis, management, and outcome. Data of the strains isolated by the microbiology laboratory were also collected, (sub)species were identified (and serovar for Salmonella enterica subsp. enterica), and antimicrobial susceptibility testing was conducted. Simultaneously, a list of the cases reported to the National Epidemiological Surveillance System by professionals of the São João University Hospital was compiled.
Categorical variables extracted from the questionnaire were analyzed mostly using the original categories provided as answer options, but regrouping was performed in some cases. Recent travel was defined as traveling outside Portugal in the 12 months prior to the diagnosis. Fever was defined as body temperature above 38 °C (or assumed when written in the records). Acute kidney injury was defined according to the 2012 KDIGO Clinical Practice Guideline [
31]. Bacteremia was defined as
Campylobacter sp./
Salmonella sp. isolation from ≥1 blood culture. Extraintestinal focal infection was defined as isolation from ≥1 samples other than feces (or other intestinal material) and blood, plus a compatible clinical picture. Antibiotic treatment was considered appropriate if the infecting strain was susceptible to ≥1 of the drugs administered. Empirical therapy was based on clinical data only, without any microbiological results. Targeted therapy was based on the results of culture and susceptibility testing. Failure was defined as persistence of symptoms and positive culture/PCR, despite appropriate therapy. Relapse was defined by recurrence of signs/symptoms and/or positive culture/PCR after initial clinical improvement and negative diagnostic tests. Outcome was assessed at the moment of discharge or death.
2.4. Statistical Analysis
Statistical analysis, including absolute and relative frequencies and hypothesis testing, was performed using IBM® SPSS® Statistics Version 29.0. Descriptive statistics are expressed as absolute frequencies and percentages for categorical variables and as means with standard deviations or medians with interquartile ranges (IQRs) for continuous variables. Comparisons between groups were performed using Pearson’s χ2 test for categorical variables (or Fisher’s exact in case of failure of the assumptions of the χ2 test). For continuous variables, after checking the assumptions of normality and homogeneity of the variances, instead of t-test and ANOVA, the Mann–Whitney U test was used for comparing two independent groups. A value of p < 0.05 was considered statistically significant. Multivariate analyses were conducted to identify factors associated with invasive disease, hospitalization, and underreporting. These analyses were performed through multiple binary logistic regression models, analyzing variables with statistical meaning in the univariate analysis (p < 0.20) and some biologically relevant or potentially confounding variables. For those variables that remained significant, crude odds ratios (ORs) were updated to adjusted odds ratios (aORs) with a 95% confidence interval (CI). The reference categories used for each independent variable are specified in each multivariate analysis results table.
4. Discussion
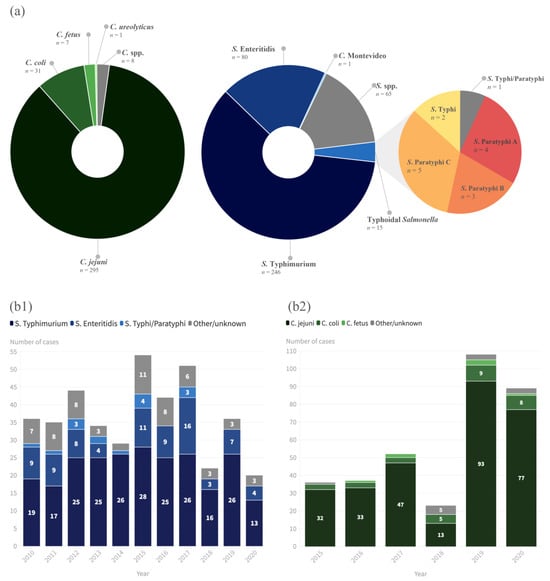
This study analyzed 10 years of NTS infections and 5 years of
Campylobacter infections in a tertiary hospital in Northern Portugal. Male sex and children were the groups predominantly affected by infection with both agents, as seen in previous national and European studies; a peak in cases of NTS between August and October was detected, but there was no clear seasonality for
Campylobacter infections, which differs from European epidemiological data [
32].
C. jejuni and
C. coli were the main species of
Campylobacter notified according to the EU One Health Zoonoses Report of 2021 [
33], and the same trend was verified in the present study, with those two species accounting for 95.3% of the
Campylobacter infections diagnosed. However, our data on
Salmonella serovars differed from those reported in the EU One Health Zoonoses Report of 2021 [
33], since the main serovar identified in our study was
S. typhimurium (84.5% of cases), followed by
S. enteritidis (8.9% of cases), versus a predominance of
S. enteritidis (54.6%) in the EU. Our data, although different from the EU’s, are similar to other studies of NTS conducted in Portugal, which showed a predominance of monophasic variants of
S. typhimurium 1,4,[5],12:i:- (26.6%), followed by Enteritidis (25.3%) and Typhimurium (14.7%) [
34]. In this study, the identification of monophasic
S. typhimurium was not feasible due to the unavailability of the requisite laboratory tests, so it was simply reported as
S. typhimurium. Additionally, not all
Salmonella specimens that were isolated underwent the typing process, which was inconclusive in other cases; therefore, some isolates were simply reported as
Salmonella sp. (not excluding TS).
In 2020, the number of NTS infections was the lowest since 2010, which could be attributed to the COVID-19 pandemic. These findings are in accordance with the EU One Health Zoonoses Report of 2021, which also showed that the notification rate decreased by 19.6% and 23.1%, with and without the data for the United Kingdom, respectively [
33]. Notably, there was an increasing trend in campylobacteriosis at our hospital. However, there are important laboratory conditions to take into account when interpreting these results. Certainly, the systematization of the use of an enteric transport medium (since 2016) and the modifications to the cultural exam protocol (incubation at 42 °C with a microaerophilic atmosphere) (since 2019) contributed to a greater recovery of
Campylobacter sp.
The symptomatic presentation, in most patients, was associated with diarrhea, fever, nausea, and vomiting. However, more than 20% of NTS infections were invasive and up to 20% were associated with AKI, which was significantly associated with hospitalizations. Immunosuppression was the only factor in the multivariate analysis that was associated with invasive disease, which is similar to other studies in the EU [
13,
14]. It was also the main risk factor for relapse in
Campylobacter infection. These data reinforce the need to consider
Campylobacter and
Salmonella infections in immunocompromised hosts, especially in cases of primary immunodeficiency for
Campylobacter and secondary immunodeficiency for
Salmonella (not only HIV infection but also inflammatory/autoimmune/neoplastic diseases that need immunosuppressive therapy). The most common form of extraintestinal focal disease was urinary infection, which can have implications in this group of patients, mainly in the differential diagnosis and choice of empiric antibiotic therapy (considering the high burden of resistance to fluoroquinolones).
Resistance to fluoroquinolones was high for both
Campylobacter (96.2% of isolates tested), NTS (69.3%) and TS (78.6%), which is compatible with the resistances found in the EU in terms of campylobacteriosis—with an average level of ciprofloxacin resistance of 64.5% for
C. jejuni and 69.6% for
C. coli—but much higher than the EU average of 14.9% in
Salmonella isolates—with the lowest levels observed in
S. typhimurium (7.6%) and the
S. typhimurium monophasic variant (8.9%)—and high to extremely high levels in
S. infantis (33.9%) and
S. kentucky (78.1%) [
33]. These higher levels of resistance to quinolones could be related to a reported higher per capita consumption of these drugs in the community sector in Portugal, compared to most countries in Western and Northern Europe [
35]. Antimicrobial susceptibility testing was performed in only half of the
Campylobacter isolates. This is not ideal since it curtails surveillance and, in cases of invasive and recurrent infections, limits knowledge regarding the antibiotic susceptibility of the isolates, which, in turn, precludes appropriate antibiotic selection.
The multidrug resistance (MDR) phenotype was present in 37.8% of NTS isolates and was significantly more common in
S. typhimurium (
p < 0.001), which is also compatible with EU data, which demonstrated a trend towards high overall MDR (22.6%,
n = 6867) among
Salmonella spp., and MDR was most frequently reported among monophasic
S. typhimurium 1,4,[5],12:i:- (78.4%,
n = 871) [
33]. The presence of MDR imposes limitations on available antimicrobial options; in this study, resistance to third-generation cephalosporins in
Salmonella was low, so it seems to be the best option for invasive disease before antimicrobial susceptibility testing is available or if it is impossible to obtain. With regard to empiric treatment of acute diarrhea, according to our data, fluoroquinolones have very limited use in our population and macrolides appear to be a reasonable alternative.
There were fifteen cases of TS, only three (20%) of which had a history of travel abroad. This contrasts with reported data for the European region, where 92.4% of TS cases in 2019 were documented as related to travel abroad [
36]. The ongoing detection of autochthonous cases, although rare, highlights the importance of serotyping every
Salmonella isolate to increase early detection, ensure adequate treatment of TS, and allow directed public health interventions. The increasing population of migrants in Portugal, including from highly TS endemic countries in South Asia [
37], could represent a direct or environmental source for TS. Systematic screening programs for migrants could help reduce the risk of autochthonous transmission and increase the overall health conditions of migrants.
The 2017 EFSA report [
32] showed a notification rate of
Salmonella infection in Portugal of 4.5 confirmed cases/100,000, which was inferior to the average in the EU (19.7/100,000). Considering the high prevalence of NTS and
Campylobacter in food and animal products and the steep discrepancy between the incidence rates of these infections in Portugal compared with other EU countries, there is a high probability of underreporting of cases. Indeed, our study showed that less than one third of the NTS and
Campylobacter infections diagnosed were reported to SINAVE. These findings are also in accordance with another study conducted in Portugal by Morgado et al. [
30] in 2015, who developed a model that generated occurrences of salmonellosis cases 93 times higher than those reported by the surveillance system (239 cases of salmonellosis reported vs. 22,201 cases estimated in 2010). The underreporting probably reflects a lack of time, unawareness about mandatory notification, and the lengthy process that is involved in notification. The information collected in the present study could be used to improve reporting by investing in simpler ways of notifying cases and in increasing awareness among healthcare professionals.
The global impact on morbidity and the economic losses of salmonellosis and campylobacteriosis are significant. Even so, the epidemiological and clinical aspects of these infections remain largely unknown, likely because many patients do not seek medical attention and, even when they do so, an empirical treatment approach to gastroenteritis is followed, without sample collection or microbiological identification. Additionally, many cases are recognized but not reported as part of an integrated surveillance network. The improvement in diagnosis and reporting could provide a wider picture of campylobacteriosis and salmonellosis and support the establishment and improvement of food safety programs, especially since these infectious agents are zoonotic.
An important limitation of this study is that it was a retrospective study and some of the data requested were not available in clinical records. Another limitation was the fact that the techniques for identification of Campylobacter changed over time, which can explain the increase in incidence from 2019 onwards. The data and conclusions from this study cannot be generalized to the Portuguese population, since the data were only collected from a specific region of the country and from patients that presented themselves to the hospital, which will likely cause a relative increase of diagnoses in children and reflect only severe cases in adults.