1. Introduction
Seaweeds play key roles in the maintenance of the biodiversity and ecological functions of marine ecosystems by improving the nutrient-rich seawater system [
1,
2,
3].
Ulva pertusa can efficiently absorb nutrients in eutrophic marine environments and can be used to ameliorate eutrophication [
4,
5]. Many studies have characterized the antimicrobial properties of seaweeds, and live
Ulva fasciata tissue can inhibit 99% of
Vibrio parahaemolyticus [
6]. Fucosterol and hexadec-4-enoic acid from
Sargassum longifolium inhibit the growth of
V. parahaemolyticus,
Vibrio vulnificus, and
Vibrio harveyii [
7]. Pang et al. [
8] found that the rapid reduction in culturable
V. parahaemolyticus in the culture of live
Grateloupia turuturu under light is caused by hydrogen peroxide or other reactive oxygen species (ROS) produced by algae. Lin et al. [
9] found that the affinity of commensal bacteria for marine microalgae was stronger than that of
Vibrio; commensal bacteria preferentially occupy the marine ecosystem, leading to
Vibrio inhibition. The composition of microorganisms in the algal micro-environment is complex and diverse, forming a unique ecological relationship with the algae. The microorganisms in the algal micro-environment can produce some unique metabolites (such as antibiotics, toxins, etc.) and release them into the algal micro-environment to enhance the disease resistance of the algae itself [
10]. Chu et al. [
11] isolated seven strains of active bacteria with an inhibitory effect on
Vibrio alginolyticus from the surface of kelp.
Vibrio abundance was negatively correlated with dissolved oxygen (DO), which indicated that increases in DO can inhibit
Vibrio growth [
12,
13,
14].
In 2020, global scallop production reached 1746.4 thousand tonnes and annual scallop production was about 7.26 thousand tonnes, which comprised 9.8% of mollusk aquaculture production [
15]. In China, the three primary scallop species cultivated in aquaculture systems are the Chinese scallop (
Chlamys farreri), the bay scallop (
Argopecten irradians), and the Japanese scallop (
Mizuhopecten yessoensis) [
16]. Among these,
Chlamys farreri is a commercially important scallop for aquaculture in China [
17]. Currently, raft culture, string-ear suspension culture, and bottom seeding culture are the main methods for breeding
C. farreri [
18]. In addition, there are also studies on the comprehensive culture of shellfish to prevent and deal with the key problem of large-scale mortality [
19], which is a representative model of green development of the aquaculture industry. By introducing filter feeding shellfish and macroalgae, and using the filter feeding of shellfish and the photosynthetic absorption of nutrients by macroalgae, the environmental pressure caused by cage culture can be reduced and the load of organic matter and nutrients can be reduced [
20]. The production of scallops in China has shown a declining tendency over the last few years, which was 2.01 million tonnes in 2017, 1.92 million tonnes in 2018, and 1.83 million tonnes in 2019 [
21]. High temperatures, the destruction of the microbial community, and the proliferation of
Vibrio can induce the mass death of Chinese scallop (
C. farreri) [
22,
23].
Vibrio is widespread in marine aquaculture systems and has a major effect on the survival of scallops [
24,
25], as it can produce extracellular toxins and enzymes that are lethal to scallops [
26,
27]. The vibriosis caused by pathogenic
Vibrio is the main cause of disease in shellfish [
28]. More than 20 pathogenic
Vibrio species have been documented to induce the mass death of shellfish in aquaculture systems [
29]. The extracellular metalloprotease (
VtpA) from
V. tubiashii is toxic to Pacific oyster (
Crassostrea gigas) larvae [
30]. Pathogenic
V. tubiashii causes the death of
Mactra chinensis [
31] and various zoonoses [
32,
33,
34,
35]. Moreover, environmental stress could affect the immune ability of scallops and the immune factor response [
36]. Superoxide dismutase (SOD) and catalase (CAT) are important members of the antioxidant reaction mechanism in organisms which can eliminate and balance the action of ROS and are important indicators to detect the immune defense ability of shellfish. Under environmental stress, scallops produce ROS and other harmful substances. SOD can decompose ROS into large amounts of H
2O
2, and the H
2O
2 can be disintegrated into H
2O and O
2 via glutathione peroxidase and CAT [
37], which can eliminate the deleterious effects of reactive oxygen species (ROS) on scallops. There is thus a need to develop strategies to prevent vibriosis and enhance the productivity of scallop cultivation.
Given that seaweeds are resistant to
Vibrio, they could potentially be used to prevent vibriosis outbreaks [
38]. In addition, our previous study [
39] showed that
U. pertusa had the most potent
Vibrio suppressive activity among eight macroalgal species, followed by
G. lemaneiformis. Therefore, we determined the optimal environmental parameters for the co-culture of macroalgae (
U. pertusa and
G. lemaneiformis) with
C. farreri through measurements of DO, pH, and
Vibrio number. This study aims to establish one co-culture system for seaweeds and scallops and to inhibit or reduce vibriosis occurrences. Generally, our findings have important implications for the development of commercially significant seaweed and will be applied to scallop and seaweed aquaculture on a large scale in the future.
3. Discussion
Seaweeds have a variety of active substances that inhibit
Vibrio species, such as polysaccharides, lipids, phenols, and terpenoids, which have strong activity [
40,
41,
42,
43]. It has also been reported that living algal bodies can inhibit
Vibrio [
6,
9]. However, little attention has been paid to the introduction of seaweeds into aquatic animal culture systems to analyze the prevention and control of vibriosis and the changes in the microbial community. At present, scallop farming is an activity of high value in the coastal countries of the world and has further become one of the main ways to utilize marine resources in these countries and regions [
44]. Mariculture shellfish, as an important economic type in aquaculture industry, brings huge benefits to the economic income of various countries [
45]. Although various breeding models of
C. farreri have been extensively investigated, disease outbreaks in
C. farreri, especially vibriosis, are still not effectively addressed [
46,
47]. Due to the gradual increase in global temperature, the proliferation of
Vibrio bacteria directly affects the physiological state of scallops and other ponderable marine organisms, leading to a serious decline in the yield and quality of aquatic products [
23]. Therefore, it is necessary to design a reasonable scallop culture model. As a metric for bacterial respiration, DO levels have been shown to be inversely correlated with
Vibrio levels [
13,
48]. Oxygen produced by seaweed promoted the inhibition of
Vibrio in the co-culture systems. Our study used co-culture involving green macroalgae (
U. pertusa) and red (
G. lemaneiformis) macroalgae with Chinese scallop (
C. farreri) to optimize reasonable co-culture systems that effectively inhibit
Vibrio by increasing DO and pH values. Here, the optimal co-culture system comprised three Chinese scallops (
C. farreri) and 30 g of seaweed (
U. pertusa/
G. lemaneiformis) in 6 L of seawater under aeration in the dark (1.25 L min
−1, 23 °C, 60 μmol photons m
−2 s
−1, 12:12 h L:D, 32 × 24 × 20 cm plastic box), as the inhibition of
Vibrio was strongest in this treatment, which has positive effects on the growth of scallops.
Vibrio inhibition was more effective under aeration in the dark than under continuous aeration (both light and dark cycles) for the
C. farreri–U. pertusa and
C. farreri–G. lemaneiformis co-culture systems, and aeration in the dark was more energy-efficient compared with continuous aeration. Therefore, the semi-closed co-culture system under aeration in the dark was used for indoor culture that could be effective for scallop culture.
Vibrio inhibition was most pronounced when the DO level reached 17.59 mg L
−1 and the pH was 9.94. Under these optimal co-culture conditions, the inhibition rate of
Vibrio was as high as 99.99% during 3 days of co-culture. We concluded that the oxygen generated by algal photosynthesis during the light cycles (without aeration) was accumulated to support the respiratory metabolism of scallops, and the increase in the DO level inhibited
Vibrio in the co-culture system. In the co-culture system with aeration in the dark, the DO and pH levels of
C. farreri–U. pertusa were slightly higher than those of
C. farreri–G. lemaneiformis during the incubation process, and
Vibrio numbers could be reduced by 6 (
C. farreri–U. pertusa) and 5 (
C. farreri–G. lemaneiformis) orders of magnitude after 3 days of co-culture. This may be because the leaf surface area of
U. pertusa was larger than that of
G. lemaneiformis, which led to a larger light-harvesting area of
U. pertusa and made the photosynthesis of
U. pertusa stronger than that of
G. lemaneiformis and its oxygen production greater. Therefore,
U. pertusa, with the same biomass, produced more oxygen than
G. lemaneiformis. The leaf surface area directly modified the exposure of single leaves, leaf arrangement, and aggregation on the shoot, which further significantly impacted the average irradiance on the leaf surface [
49,
50]. Nevertheless, under continuous aeration, the oxygen produced by algal photosynthesis during the light cycles was driven to the air due to aeration, and the oxygen could not be accumulated, resulting in the failure of DO reaching a concentration that could inhibit
Vibrio. Furthermore, we first reported the co-culture conditions of aeration in the dark, which not only ensured that the respiration of scallops was unaffected without oxygen supply from seaweed photosynthesis but also allowed oxygen accumulation to improve DO levels during the day, achieving a better
Vibrio inhibition effect.
The nutrients in seawater mainly include NH
4-N, NO
3-N, NO
2-N, and PO
4-P [
51,
52]. As a harmful substance mainly to marine animals, NH
4-N is produced by the decomposition of organic matter such as aquatic animal excreta, biological carcasses, and bait residues from microorganisms [
53]. It can affect the activity of enzymes in aquatic animals, and then affect their development and growth [
54]. Widman et al. [
55] indicated that ammonia nitrogen was the most toxic nitrogen-containing waste to Juvenile Bay Scallops (
Argopecten irradians) in the bay. As an intermediate product of nitrification and denitrification, NO
2-N could inhibit the activity of important metabolic enzymes in organisms when it is enriched to a certain extent, which leads to metabolic dysfunction, physical decline, and large outbreaks of disease and death in shellfish [
56]. The high content of phosphorus in seawater will lead to eutrophication of seawater and cause red tides, which will affect the growth of scallops [
51]. The low content of phosphorus will affect the growth of phytoplankton in seawater, resulting in a lack of food for scallops in the breeding process, thus affecting breeding benefits [
51]. In the optimized co-culture systems of CU and CG, the NH
4-N, NO
3-N, NO
2-N, and PO
4-P concentrations increased in the first 24 h of the culture period and then decreased gradually below pre-culture concentrations. Nutrient concentrations under aeration in the dark were consistently lower than those under continuous aeration. This might indicate that continuous aeration limits nutrient uptake by algae. The applied
U. pertusa and
G. lemaneiformis could absorb NH
4-N, NO
3-N, NO
2-N, and PO
4-P and reduce eutrophication in seawater in the co-culture system under aeration in the dark.
SOD and CAT are momentous antioxidant enzymes in organisms [
57], and their content changes could reflect the oxidative stress level of scallops and indicate the immune defense ability of shellfish at the biochemical level [
58]. Under aeration in the dark, the SOD and CAT activities increased over 3 d of co-culture, which indicates that
C. farreri exhibited positive immunological responses to aeration in the dark. High DO and pH levels induced stress responses in scallops, and the increase in SOD activity promoted ROS scavenging, which generated large amounts of H
2O
2. The H
2O
2 was then disintegrated into H
2O and O
2 via glutathione peroxidase and CAT [
37], which eliminated the deleterious effects of ROS on scallops. Over 3 d of co-culture, PK activities in the adductor muscles, mantle, and gills increased, and LDH activities decreased. PK activities were higher in the adductor muscles than in the mantle and gills; this likely stems from the fact that the adductor muscles require more energy than the mantle and gills given that they are responsible for the opening and closing of the shell [
59]. In conclusion, variation in the activities of these four enzymes indicates that the immune and metabolic activities of
C. farreri were enhanced in the optimal co-culture system.
Bacteria are critical ecosystem drivers in both aquatic and terrestrial ecosystems and are highly diverse and complex organisms that underpin biogeochemical cycles across diverse ecosystems [
60,
61,
62]. In the optimal co-culture system, the abundance of
Vibrio decreased in the seawater and seaweed epiphyte samples during the 3 d co-culture period. Patescibacteria are known to have minimal biosynthetic and metabolic pathways and are able to attach to multiple hosts for just long enough to loot or exchange supplies [
63]. We speculate that its simple metabolic pathways and low-energy attachment mechanism facilitate adaptation to the optimal co-culture system; this also explains the increase in the relative abundance of Patescibacteria over the 3 d co-culture period. Similarly, many studies have shown that the relative abundance of ultra-small Patescibacteria lineages frequently exceed those of other bacteria in diversity surveys [
63,
64,
65,
66,
67]. Proteobacteria was the second largest phylum of hydrogenogenic CO oxidizers [
68], and the decrease in Proteobacteria after 3 days allowed the scallops and seaweed to maintain a good physiological state in the optimal co-culture system. Given that some
Bacteroides are aerotolerant [
69], the relative abundance of
Bacteroideta significantly increased in the co-culture system with high DO levels. Based on the BugBase phenotypic prediction, the relative abundance of aerobic bacteria increased, whereas that of anaerobic bacteria decreased after 3 days of culture. This may be related to the increase in DO levels in the co-culture system. A high proportion of potentially pathogenic microorganisms appeared in CUE0, suggesting that the potential pathogenic microorganisms might come from
U. pertusa. Most importantly, the potential pathogenicity of the microorganisms attenuated in the optimal co-culture systems after 3 days of culture, which further proved the effectiveness of our designed co-culture systems. Thus, the co-culture of seaweeds and scallops on large scales could inhibit
Vibrio and promote scallop growth.
4. Materials and Methods
4.1. Scallops, Seaweeds, and Vibrio Species
Live C. farreri was obtained from a fish market in Qingdao, Shandong, China; healthy scallops (shell height: 3–5 cm; wet weight: 32 ± 5 g) were used in the experiments. After washing the scallops with seawater to remove debris, three scallops were placed in each plastic box (32 × 24 × 20 cm, a total of 9 plastic boxes) with 6 L of freshly filtered seawater (through 0.44 μm microporous membranes) aerated at 1.25 L min−1 at 23 °C. Ulva pertusa (containing holdfasts and fronds with irregular holes) was collected from the intertidal zone in Huiquan Bay (120°20′32.6″ E, 36°3′24.0″ N), Qingdao, China; G. lemaneiformis was obtained from a farm in Putian, Fujian, China. All the seaweed samples were washed with fresh seawater and pre-cultured for 3 d in a plastic box (32 × 24 × 20 cm) at 23 °C; 50% fresh seawater was exchanged daily under natural illumination. In the experiments, a total of three Vibrio strains maintained in the laboratory were utilized: V. tubiashii, V. splendidus, and Vibrio sp. MM5. V. tubiashii was previously isolated from Argopecten irradians larvae in 1989, V. splendidus was obtained from the liver of diseased Chinese white shrimp (Penaeus chinensis) in 1990, and Vibrio sp. MM5 was isolated from moribund clam (Meretrix meretrix) in 2007.
4.2. Determination of Vibrio Quantity, DO, and pH
The protocol for inoculating Vibrio suspensions was as follows: V. tubiashii, V. splendidus, and Vibrio sp. MM5 stored in 2216E agar slant culture medium were streaked on TCBS plate medium and cultured at 23 °C for 24 h. Subsequently, the single colonies with smooth edges were selected, re-streaked, and cultured for 24 h at 23 °C. The single colonies with smooth edges were then inoculated into 900 mL of 2216E liquid medium and underwent shaking in an incubator at 150 r min−1 (23 °C, 24 h). Next, the Vibrio precipitate was collected by centrifugation at 1776× g (23 °C, 20 min) and thoroughly resuspended using 10 mL of sterilized seawater (0.44 μm filter membrane, high-temperature autoclaving) to prepare three Vibrio suspensions.
To determine the number of
Vibrio, 1 mL of stirred seawater sample was diluted 1–6 times (m) and 100 μL of diluted seawater was coated on the TCBS selective medium (Solarbio, Beijing, China). The inoculated TCBS plate was then incubated at 23 °C for 48 h, and the number of
Vibrio colonies (n) was counted. All of the above operations were performed on an ultra-clean workbench. Three cycles of TCBS medium were used for each sample.
The DO and pH levels of the seawater were measured using a FireSting-O2 m (Beijing Ecotech Ecological Technology Ltd., Beijing, China) and METTLER TOLEDO pH meter (FE20, Shanghai Mettler Toledo Ltd., Shanghai, China), respectively. Air in the co-culture was provided by an air pump (Zhongshan SOBO, Electric Appliance Co. Ltd., Zhongshan, China). Data on the number of Vibrio, DO level, and pH level were measured every 12 h during the 72 h culture period.
4.3. Experimental Design
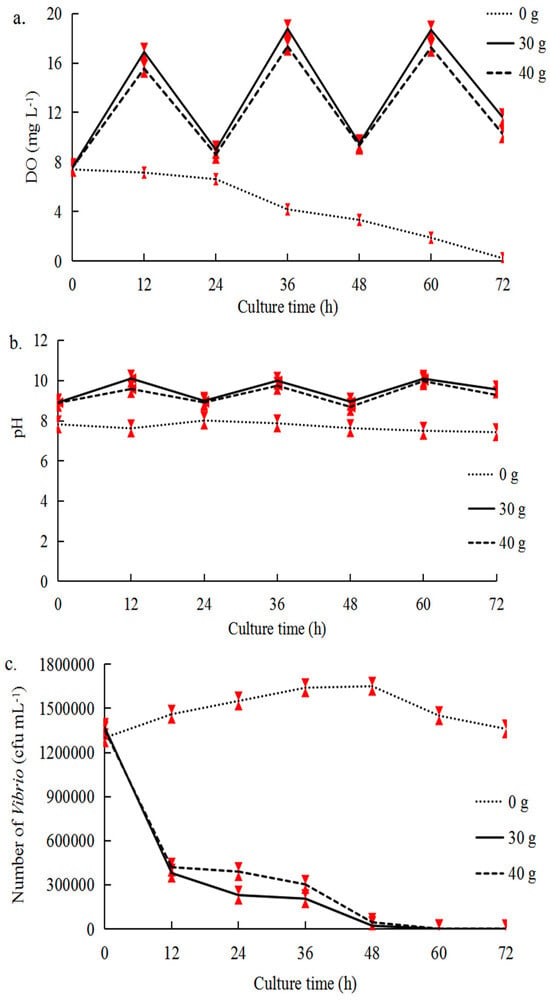
We conducted a series of experiments to determine the optimal co-culture conditions for inhibiting
Vibrio (
Table 1). Firstly, we aimed to determine the optimal amount of seaweed for
Vibrio inhibition in Experiment A. In total, 0 g, 30 g, and 40 g of fresh
U. pertusa were added to each plastic box (32 × 24 × 20 cm) containing 6 L of seawater (without scallops), and 0 g
U. pertusa served as the control group. The
Vibrio suspensions (about 10
7 cfu mL
−1) were then added into each plastic box. Comprehensively considering the alterations in the three factors (
Vibrio number, DO value, and pH value) and the effect of mutual shading by seaweed in the plastic boxes, the optimal algae density that produced the most pronounced effect of decreasing the number of cultivable
Vibrio was determined. Secondly, in Experiment B, we aimed to determine the optimal aeration conditions and scallop number that inhibited
Vibrio bacteria and promoted scallop growth. The initial concentration of
Vibrio suspensions was about 10
7 cfu mL
−1. We selected three scallops and 30 g
U. pertusa for the co-culture to explore the optimal aeration conditions. The co-culture system of
C. farreri–U. pertusa was subjected to three sets of aeration conditions by using air stones to ad air: no aeration, continuous aeration (1.25 L min
−1) during light and dark cycles, and aeration (1.25 L min
−1) during the dark cycles and the absence of aeration during the light cycles. Under the optimized ventilation conditions explored above, three, four, and five scallops (
C. farreri) were used to determine the optimal number of scallops with 6 L of seawater and 30 g or
U. pertusa. Then, in Experiment C, we evaluated the efficacy of the optimal amount of seaweed and scallops and the aeration conditions identified in Experiments A and B for the co-culture of
C. farreri with
U. pertusa and
G. lemaneiformis. Control and experimental groups (with the addition of
Vibrio suspensions, about 10
7 cfu mL
−1) were used to determine the extent to which these optimal conditions inhibited
Vibrio. Finally, in Experiment D, we evaluated the effectiveness of applying these optimal conditions to a normal aquaculture environment (without adding
Vibrio suspensions) by measuring the enzyme activities of the scallops, nutrient salts, the abundance of
Vibrio, and microbial diversities.
In Experiment A, B, and C, the
Vibrio number, DO value, and pH value were measured every 12 hrs until the end of the 72 hrs. Details on all experiments (Experiment A, B, C, D) are provided in
Table 1. There were three replicates in each group, and we used 12 h:12 h light/dark cycles and 60 μmol photons m
−2s
−1 (LED).
4.4. Nutrient Detection
In Experiment D, all groups were pre-cultured for 1 h under the same culture conditions. A total of 200 mL of seawater samples was collected at 24-h intervals until the end of the 72-h period, and seawater was stirred to insure homogenization before sampling. These seawater samples were used to determine the ammonia nitrogen (NH
4-N), nitrate nitrogen (NO
3-N), nitrite nitrogen (NO
2-N), and inorganic phosphorus (PO
4-P) content. Measurements of these nutrients were conducted according to national standards for marine sampling [
70]. NH
4-N was measured using the indophenol blue method, NO
2-N was measured using
N-naphthylethylenediamine dihydrochloride, and PO
4-P was measured using the phosphorus-molybdenum blue method [
71,
72]. NO
3-N was measured using the cadmium column reduction method with a reduction column (LS_3105) obtained from Qingdao Anlixin Trading Co., Ltd. (Qingdao, China).
The NH
4-N, NO
2-N, NO
3-N, and PO
4-P concentrations were measured in Experiment D, and they were calculated using the following formulas (
Figure S1):
4.5. Enzyme Activity Assays
In Experiment D, the activities of SOD, CAT, PK, and LDH in the scallops were determined to evaluate the immune and metabolic activities of the scallops. Crude enzyme extracts were obtained from the adductor muscles, mantles, liver pancreas, and gills, and enzyme activities were determined at 0 h and 72 h during the co-culture period. All three scallops from each box were collected into a sterilized 2 mL centrifuge tube as a replicate, and each group was replicated with three plastic boxes. The extracted crude protein was obtained using a BCA kit (Solarbio, Beijing, China) per the manufacturer’s instructions. The SOD and CAT activities of the adductor muscles, liver pancreas, and mantle were measured, and the PK and LDH activities of the adductor muscles, mantle, and gills were measured. The activities of all enzymes were measured using enzyme detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) per the manufacturer’s instructions.
4.6. Microbial Diversity Analysis
In Experiment D, microorganisms in the seawater and on seaweeds (epiphytic microorganisms) were collected before and 3 d after culture, and details on the 26 microbial samples are summarized in
Table S2. The microorganisms from the seawater of each plastic box were filtered through 0.22 μm microporous membranes. Epiphytic microorganisms on the seaweed surface were sampled by adding 30 g of fresh seaweed into a box with 1 L of sterilized seawater (high-temperature autoclaving) with sterilized glass beads (ca. 2 mm in diameter). They were then shaken in an incubator at 1776×
g (60 min, 23 °C), and the seawater was filtered through 0.22 μm microporous membranes. The samples were then placed in sterilized centrifuge tubes, snap-frozen in liquid nitrogen for 3–5 min, and stored at −80 °C. All 26 samples were plated on TCBS plates before filtration to determine
Vibrio number, and each sample used three TCBS plates as replicates.
Total genomic DNA was extracted from the 26 samples using the TGuide S96 Magnetic Soil/Stool DNA Kit (TIANGEN Biotech, Beijing, China) per the manufacturer’s instructions, and the DNA concentration and purity were detected using a NanoDrop 2000 UV-Vis spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The V3-V4 hypervariable region of the bacterial 16S rRNA gene was amplified using the primer pair F: 5′-ACTCCTACGGGAGGCAGCA-3′ and R: 5′-GGACTACHVGGGTWTCTAAT-3′; the contents of the PCR are shown in
Table S1. PCR products were purified using the Omega DNA purification kit (Omega Inc., Norcross, GA, USA). The amplicon library was paired-end sequenced (2 × 250) on an Illumina NovaSeq 6000 platform (Beijing Biomarker Technologies Co., Ltd., Beijing, China). Raw data were filtered using Trimmomatic v0.33 [
73]; primer sequences were removed using Cutadapt v1.9.1 [
74] to obtain the clean reads. The DADA2 method [
75] in QIIME2 v 2020.6 [
76] was used to denoise the data after quality control to generate amplicon sequence variants (ASVs), and low-abundance ASVs (less than 0.005%) were removed. Taxonomic annotations of ASVs were determined using the Bayesian classifier algorithm and the SILVA database (
http://www.arb-silva.de (accessed on 21 July 2023)) [
77]. QIIME was used to determine the abundance of each species in samples and generate the species distribution histogram based on the composition of ASVs.
Alpha diversity was analyzed using QIIME2, and species diversity, the Ace, Chao1, Shannon, and Simpson indices were calculated in R software 4.1.0. BugBase [
78] was used to predict the organism-level coverage of functional pathways within complex microbiomes as well as biologically interpretable phenotypes. BugBase first normalized operational taxonomic units through prediction of the 16S copy number, and microbial phenotypes were predicted using precalculated files with a threshold of 0.01.
4.7. Statistical Analysis
The statistical analysis was conducted utilizing a completely randomized design with three replicates. For the statistical analysis, SPSS version 21 was utilized. To compare the means, one-way ANOVA and Tukey’s HSD’s multiple range tests were employed with a significance level of p < 0.05.