1. Introduction
Inner ear disorders resulting in hearing loss are a problem worldwide, and the prevalence is increasing as the population ages [
1,
2,
3]. Sensorineural hearing loss is a debilitating condition that results in social isolation, cognitive decline, and even increased mortality [
4,
5,
6,
7,
8,
9]. Treatment of sensorineural hearing loss currently remains limited to auditory prosthetic devices such as hearing aids and cochlear implants.
Developing therapies to treat hearing loss remains difficult because many barriers to therapeutic delivery into the inner ear exist [
10,
11,
12,
13,
14]. Systemic delivery is limited by the blood labyrinth barrier, akin to the blood brain barrier, which is permeable only to select molecules due to tight junctions between endothelial cells and pericytes and perivascular macrophages surrounding the basement membrane of the endothelial cells [
15,
16,
17,
18,
19]. The cochlea is encased in dense otic capsule bone, in a location deep within the temporal bone, which also complicates local therapeutic delivery. Intratympanic injection can be done in clinic by an otolaryngologist, but this has limited penetration into the cochlea due to the presence of the round window membrane, which causes a 1000-fold decrease in drug concentration between the middle ear and inner ear, as well as a gradient in drug concentration from the base decreasing to the apex [
20].
Other local drug delivery methods to the inner ear include infusion directly through the round window membrane or into the lateral or posterior semicircular canal. These both require an outpatient surgical procedure but are much more efficient at delivering therapeutics into the inner ear as they directly access the inner ear fluid chambers. Round window infusion is accessible with a surgical approach through the ear canal. The infusion initially enters the scala tympani and carries a risk of sensorineural hearing loss due to direct infusion into the cochlea [
21]. This technique has shown efficacy with partial restoration of hearing in one genetic form of human hearing loss [
22,
23]. Infusion into the lateral or posterior semicircular canal is another technique used to deliver therapeutics directly into the inner ear space. Lateral semicircular canal infusion has been proposed for use in early clinical trials. Posterior semicircular canal (PSCC) infusion has mainly been used in rodent models such as mice [
24,
25,
26,
27]. Either lateral or posterior semicircular canal infusion is easily translatable to human patients as it requires a simple mastoidectomy, which is a routine outpatient surgery done for chronic ear infections or cochlear implantation. Though PSCC infusion of therapeutics has been performed in animal models and shown gene transduction in the inner ear, the immediate distribution of injected material within the cochlea after PSCC infusion has not been well studied. Recent studies prove that PSCC infusion initially distributes throughout the cochlear perilymphatic space. Thus, the basolateral surfaces of hair cells and supporting cells within the organ of Corti would be exposed to the perfusate [
28]. As the semicircular canal infusion technique does not directly access the cochlea, an advantage of this technique is a decreased risk of sensorineural hearing loss, and work done on mouse models show minimal impact on vestibular function [
21,
28,
29].
The delivery systems, or delivery vehicles, under investigation for inner ear drug and gene therapy are vast and varied [
30,
31]. Viral vectors are typically used to deliver genetic material in animal models, most commonly adeno-associated virus [
32,
33]. They are minimally pathogenic and efficient at transduction in the inner ear; however, they have limited capacity for genetic material, 4.7 kb for a single AAV vector, and are limited in the capacity to deliver therapeutics other than in the form of nucleic acids [
34]. One alternative to viral vectors are nanoparticles. Nanoparticles are not pathogenic and can be made with a wide variety of materials. They are versatile in their ability to deliver small-molecule therapeutics to macromolecular therapeutics, including nucleic acids and proteins [
14,
31,
35,
36]. They can be targeted to specific cell types and be delivered intracellularly via receptor-mediated endocytosis [
37,
38,
39]. For example, using ex vivo inner ear rodent animal models, gold nanoparticles have been shown to be targeted to hair cells and deliver Myosin XVa plasmid constructs intracellularly so that the protein can be expressed at the appropriate location, the tips of hair cell stereocilia [
40,
41]. However, one potential downside to using nanoparticles in the cochlea is their mass. When they settle, there may be adverse effects on the vibratory properties of the organ of Corti.
To address this point, we characterized the distribution of nanoparticles after PSCC infusion as well as their effect on cochlear mechanics. We chose to use gold nanoparticles rather than other types of nanoparticles because they have a high refractive index and can be detected using optical coherence tomography imaging [
42]. Furthermore, they have intrinsic two photon fluorescence properties [
43,
44,
45,
46,
47,
48] and can be readily functionalized with fluorophores or conjugated with targeting agents or therapeutics including peptides, proteins, or nucleic acids [
39,
49,
50]. Finally, they are among the heaviest of nanoparticles and thus provide a rigorous test for mass loading of the organ of Corti.
3. Discussion
Delivering therapeutics to the cochlea is already happening in clinical practice. Intratympanic injection of steroids into the middle ear with diffusion into the inner ear is routine treatment for sudden sensorineural hearing loss. Round window and lateral semicircular canal infusion of AAV is being performed for gene therapy clinical trials in otoferlin deficiency, which causes an auditory neuropathy spectrum phenotype that results in severe to profound sensorineural hearing loss [
22,
23]. Here we show that nanoparticles offer another approach to intracochlear drug delivery. We found that gold nanoparticles ranging from 52–102 nm at high 10
12–10
13 particles/mL concentrations can be infused via the PSCC and perfuse the entire cochlear perilymphatic space in mice without disrupting cochlear mechanics. This means that neither the mass of the nanoparticles nor the infusion technique adversely affects a key aspect of cochlear physiology, the non-linear vibratory characteristics in response to sound. Our cochlear mechanics results at 1 h after nanoparticle perfusion demonstrate that nanoparticles are a safe therapeutic delivery system when using direct inner ear infusion and pave the way perhaps for not just treating and rescuing severe to profound hearing loss, but also for treating milder forms of hearing loss and other types of inner ear disease that may not have severe hearing loss.
Direct inner ear infusion is an efficient method to deliver therapeutics into the cochlea as the drug directly enters the inner ear space [
11]. Other techniques for inner ear drug delivery include intratympanic and systemic delivery methods. The round window membrane significantly limits the amount of drug in the inner ear to 1/1000 of the drug concentration delivered to the middle ear [
58]. The blood labyrinth barrier also significantly limits drug entry from the bloodstream into the cochlear fluid compartments, with higher risks of systemic side effects [
17]. A few studies have shown some efficacy with nanoparticle therapeutic delivery to the rodent cochlea. However, a number of these studies were performed ex vivo or attempted delivery that required the therapeutic to traverse the round window membrane [
30,
31,
59].
We provide a method for direct intracochlear nanoparticle delivery. Though we do not deliver a therapeutic with our nanoparticles, we show that nanoparticles enter the cochlear perilymphatic space with PSCC infusion, and they eventually reach the inner hair cell region of the organ of Corti. This is where therapeutics need to reach to be effective in treating hearing loss. The mechanism by which this occurs requires further study but likely occurs via transcytosis through supporting cells in the basilar membrane. Transcytosis is a common mechanism by which nanoparticles can cross cell layers, such as in the blood brain barrier [
39], and is a potential mechanism by which nanoparticles enter through the basilar membrane into the organ of Corti from the perilymphatic compartment.
Limitations of our study include the lack of long-term cochlear mechanics and hearing data. Though PSCC infusion can be performed as a survival surgery in mice, opening the bulla to expose the cochlea is too invasive to allow for survival surgery, thus limiting the amount of time that cochlear mechanics recordings can be made. We were more interested in whether any changes in cochlear mechanics would occur after gold nanoparticle infusion due to the mass of the gold. Thus, auditory brainstem responses (ABRs) as a surrogate for hearing status were not measured. However, it is reasonable to infer based on previous work that unchanged cochlear mechanics would also result in unchanged ABRs in these wild-type CBA/CaJ mice. For example, we previously showed that artificial perilymph infusion via the PSCC does not change cochlear function or auditory brainstem responses (ABR) at 1 h post-infusion in these wild-type mice, and other studies also show that tube insertion and slow PSCC infusion of 1–2 µL do not affect ABR [
28,
29]. Measuring ABR would be important to investigate with future work utilizing nanoparticles delivering a therapeutic, as outcomes could vary with the hearing loss model. For example, intact cochlear mechanics could still result in hearing loss on ABRs in auditory neuropathy spectrum phenotypes resulting from impaired synaptic transmission between the inner hair cell and afferent auditory nerve fibers. Lastly, the long-term deposition of nanoparticles in the inner ear, and whether some of the nanoparticles migrate to the intracranial space through the cochlear aqueduct, is a topic of future study.
Development of therapeutic strategies to treat or reverse hearing loss, and delivery of therapeutics into the inner ear, is becoming increasingly important as our knowledge of causative mechanisms for hearing loss grows. Intracochlear therapeutic delivery with PSCC nanoparticle infusion can serve this purpose. Future work can include targeting the nanoparticles to different regions in the organ of Corti or engineering them to deliver a variety or even a combination of therapeutic classes, including small molecules, proteins, and nucleic acids, which could be required to treat the multitude of etiologies for hearing loss and inner ear disease.
4. Materials and Methods
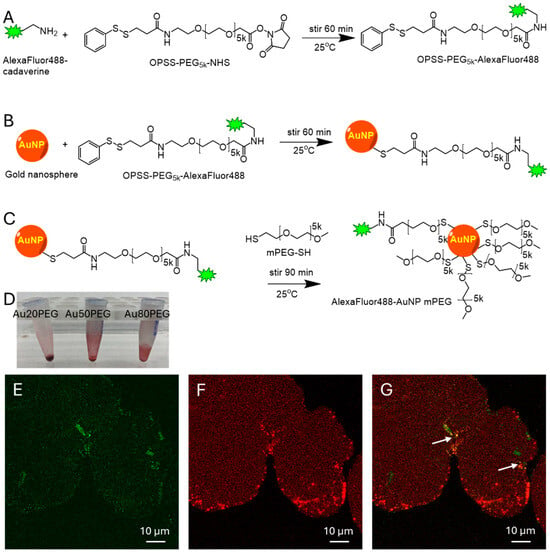
Nanoparticle Synthesis. A fluorescent AlexaFluor488-cadaverine (AF488) (ThermoFisher Scientific, Waltham, MA, USA, used as received) was conjugated via an amide bond to a functionalized polyethylene glycol (PEG) polymer PEG5k-OPSS (Creative PEGWorks, Chapel Hill, NC, USA, used as received) (
Figure 1A). Excess AF488 was separated from the PEGylated AF488 using a 3 kDa filter cutoff (Millipore Amicon Ultra, Millipore Sigma, Burlington, MA, USA) in microcentrifuge at 15,000 rpm. Spherical gold nanoparticle (NP) cores of 20 nm, 50 nm, and 80 nm sizes were purchased from nanoComposix (San Diego, CA, USA, used as received) and first stirred with AlexaFluor488-PEG5k-OPSS to create a covalent disulfide linkage on the surface of the gold NPs (
Figure 1B). Then, excess methoxy-PEG5k-SH (Laysan Bio, Arab, AL, USA, used as received) was added to the reaction to form covalent disulfide bonds on any further reactive binding sites on the gold NP core (
Figure 1C) to stabilize the NP and prevent aggregation. The NPs were centrifuged at 15,000 rpm to form a pellet, which was washed three times to remove excess PEG5k, and resuspended in artificial perilymph (140 mM NaCl, 2 mM KCl, 2 mM MgCl
2, 2 mM CaCl
2, 20 mM HEPES with pH 7.4 and 303–307 mOSm/kg) at a high concentration in preparation for PSCC infusion. This created fluorescently labeled gold NPs, which are diagramed in
Figure 1. The NP was stable in physiologic salt concentrations.
Nanoparticle Characterization. The NP size and charge of the 20 nm, 50 nm, and 80 nm gold cores containing fluorophore and mPEG, named Au20PEG, Au50PEG, and Au80PEG, were measured using a Brookhaven Instruments ZetaPALS with dynamic light scattering and zeta potential to determine hydrodynamic diameter and surface charge, respectively. Values are shown in
Table 1 and are consistent with similar types of gold NPs previously reported [
39,
49]. We confirmed these NPs were fluorescent using a fluorimeter, epifluorescent microscope, and 2-photon fluorescent microscopy. Though NP concentration can be determined using an UV-visible absorbance technique with an Eppendorf BioSpectrometer based on the gold surface plasmon resonance using absorption and Beer’s law at the maximal absorption wavelength (size dependent, 520–550 nm), the high concentration of our nanoparticle solution in artificial perilymph is out of range of the standard curve and prevents the use of this method. Thus, we calculated the estimated concentration shown in
Table 1 using the volume and concentration of gold NPs from the stock solution used in the synthesis, and the final volume of the concentrated functionalized nanoparticle solution.
Mouse Surgery. All experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at the University of Southern California, and all methods were performed in accordance with the relevant guidelines and regulations between 2021 and 2024. We used CBA/CaJ (JAX#: 000654, The Jackson Laboratory, Bar Harbor, ME, USA) wild-type male and female mice with normal hearing age 7–10 weeks [
60,
61]. Mice were anesthetized using intraperitoneal 80–100 mg/kg ketamine and 5–10 mg/kg xylazine. No invasive procedures were performed until surgical anesthesia was reached. Anesthesia depth was assessed at 15-min intervals with supplemental anesthetic doses administered to maintain anesthesia. An electric heating pad maintained the animal’s body temperature at 38–39 °C. We then proceeded with a post-auricular approach to the middle ear bulla, which was opened to reveal the otic capsule bone and round window membrane [
62]. Once the experiment was completed, the mouse was euthanized while under anesthesia.
PSCC Infusion Technique. After opening the middle ear bulla to expose the mouse cochlea, the PSCC was exposed and the PSCC infusion procedure was completed as previously described [
25,
26,
28]. Briefly, a canalostomy was made in the PSCC with the tip of a 28-gauge needle. The infusion tubing is a 110 µm polyimide tubing (MicroLumen, Oldsmar, FL, USA) attached inside a 280 µm polyethylene tubing (SIMS Portex Ltd., Hythe, Kent, UK) with Krazy Glue, which was placed over the needle of a 10 µL gastight Hamilton syringe (Hamilton, Reno, NV, USA). The infusion tubing was filled with gold nanoparticle solution at the concentration listed in
Table 1 suspending in artificial perilymph (140 mM NaCl, 2 mM KCl, 2 mM MgCl
2, 2 mM CaCl
2, 20 mM HEPES with pH 7.4 and 303–307 mOSm/kg). This was a high concentration of gold nanoparticles based on what has previously been used to visualize nanoparticles within the cochlea using OCT [
28,
63]. The infusion tubing containing the gold nanoparticle solution was placed into the PSCC canalostomy and was sealed with histoacryl tissue glue (Tissue Seal, LLC, Ann Arbor, MI, USA). 1 µL of nanoparticle solution in artificial perilymph was infused into the PSCC at 0.5 µL/min over 2 min using a one-channel programmable syringe pump (New Era Pump Systems, Inc., Farmingdale, NY, USA). For two-photon imaging experiments, another 1 µL was delivered 10 min after the initial infusion to increase the concentration of nanoparticles delivered for fluorescence visualization and minimize infusion volume and pressure at a single time point.
Optical Coherence Tomography and Vibrometry Measurements. Our custom-built OCT system has been previously described [
60,
63,
64]. We have built multiple versions of this system. The one used for this work had a swept-laser source with a center wavelength of 1306 nm, 90.4 nm bandwidth, and sweeping at 100 kHz. The resulting axial resolution, assuming a refractive index of 1.4 and a Hann window for spectral shaping, was 13.5 µm. The sample arm of the OCT interferometer is integrated into a stereo microscope, Zeiss Stemi-2000. A 2D voice-coil mirror setup for telecentric scanning through an objective lens provided a lateral resolution of 9.8 µm.
OCT images were obtained at baseline after exposing the cochlea, after infusion tubing insertion into the PSCC, during and at time intervals immediately after PSCC infusion up to approximately 60–75 min after nanoparticle infusion. Sound stimuli for vibrometry measurements were delivered in a similar manner as previously described in an open field configuration using an Etymotic ER2SE earbud and consisted of pure tones with frequencies ranging from 0.5–15 kHz in 0.5 kHz steps and levels ranging from 10–80 dB SPL in 10 dB steps. Each stimulus duration was 100 ms. Basilar membrane vibrometry measurements were taken at baseline following surgical opening of the bulla, after performing a PSCC canalostomy and inserting tubing containing nanoparticle solution, immediately following infusion of the nanoparticle solution, up to approximately 60–75 min after NP infusion, and immediately following euthanasia of the mouse at the conclusion of the experiment. The characteristic frequency (CF) was identified as the frequency with the largest vibratory response to the lowest stimulus intensity that produced a vibratory response above the noise floor. Cochlear gain was calculated as the difference in vibratory sensitivity (displacement normalized to the sound intensity) to 80- and 20-dB SPL stimuli at the CF. Q10dB was calculated at 40 dB SPL. The gain, CF, and Q10dB were calculated from basilar membrane vibrometry recordings prior to (0 min), after PSCC infusion of the solution (at approximately 5–15 min referred to as immediately after infusion and 60–75 min referred to as 1 h after infusion), and after euthanasia of the mouse. These data were analyzed in MATLAB. All vibrometry experiments were carried out on the left ear.
Two-Photon Optical Coherence Microscopy (2P OCM). After PSCC infusion of Au50PEG nanoparticles, the mouse was kept under anesthesia for 7 h and then euthanized. The cochlea was excised, and the otic capsule bone was scraped away to be able to visualize the organ of Corti directly. The freshly excised cochlea was held in place on a homemade glass-bottom chamber with Histoacryl glue (Tissue Seal, LLC, Ann Arbor, MI, USA) and imaged using our two-photon optical coherence microscopy system detailed below. These were compared with cochlea from control mice.
Our two-photon and optical coherence microscopy system has previously been described [
65,
66]. Briefly, two-photon excitation was accomplished by scanning a 150-mW femtosecond pulsed laser at 930 nm (Chameleon Ultra II, Coherent, Saxonburg, PA, USA) across the sample through the trinocular port on a Nikon upright microscope (Nikon Instruments Inc., Melville, NY, USA) with a Nikon 16× (0.8 NA) water-immersion objective. Fluorescence from the sample was collected through the objective and separated from the excitation beam path with a long-pass dichroic mirror with a cutoff wavelength of 735 nm. The red and green fluorescence channels were separated using another long-pass dichroic mirror with a cutoff wavelength of 585 nm. A final filter was added to the green channel that further reduced the spectrum of green fluorescence to a bandwidth of 40 nm centered at 500 nm. The detectors used in both channels were hybrid photodetectors (R11322U-40, Hamamatsu, Japan) with variable gain settings that allowed control of relative brightness between the fluorescence detected from the two channels.
Two-photon and optical microscopy z-stacks were acquired simultaneously with customized software. The field of view was 134 μm in diameter. Volumes were acquired using 1 µm steps in the z direction for a total of 50 steps. Images were imported into ImageJ for analysis.
Two-Photon Microscopy. Gold nanoparticle solution on a slide was imaged using an inverted microscope (Leica SP-8X MP, Leica Microsystems, Deerfield, IL, USA) with an excitation wavelength of 930 nm and emission wavelengths of 494–529 nm and 588–642 nm for the green and red channels, respectively. A 20× (0.75 N.A.) objective was used for imaging. The images were exported into ImageJ for analysis.