Uranium is an important material basis for the development of the nuclear industry. It is very important for the stable development of the nuclear energy industry and nuclear industry in general [
1,
2,
3]. It plays an important role in ensuring national energy security, promoting scientific and technological progress and achieving sustainable development. The purification and conversion of uranium is a key step in the preparation of nuclear fuel elements, which converts natural uranium ore into high-purity uranium compounds. However, a large amount of refractory uranium-containing purified dissolved slag will be produced during the purification and conversion of uranium [
4,
5,
6]. As part of uranium-containing radioactive tailings, the main components of the uranium-purification dissolution residue not only include silicate, silica, and other heavy metal compounds from the mine, but they also include emulsified sludge in the internal circulation liquid from uranium-purification production. The main components of the emulsified sludge are the emulsification products of organic phase extractants. The sources are complex and vary greatly, and it is difficult for them to be recovered by conventional means [
6,
7,
8]. Before the slag discharge requirements are met, only temporary measures can be taken to deal with such uranium-containing tailings, and the uranium resources cannot be effectively recovered. At the same time, long-term open-air stacking will cause potential harm to the ecological security of the surrounding environment and ultimately endanger human health. In addition, China’s uranium resources are in short supply, and its external dependence is increasing year by year. Therefore, the recovery of uranium from uranium-containing tailings can solve the problem of the uranium resource shortage and environmental pollution.
At present, the main methods for recovering uranium from such complex and difficult-to-treat uranium-containing waste residues are oxidative leaching, acid-alkali combined leaching, and roasting-acid leaching. The oxidation leaching method [
9,
10] uses oxidants such as MnO
2 and H
2O
2 to oxidize U
4+ to U
6+. U
6+ has better solubility in common leaching agents than U
4+ and is easier to leach. Its characteristics improve the leaching rate. However, the addition of a large number of strong oxidants will increase the cost and secondary environmental pollution. At the same time, more ions will enter, which is not conducive to the subsequent treatment and purification of the leaching solution. The acid-alkali combined leaching method [
11,
12,
13] is the first to use an alkaline solution to selectively destroy the gangue structure of the ore. Cracks and pores are formed, and the method of extracting uranium from ore by an acid-leaching agent is used again. The roasting-leaching method [
14,
15,
16] first destroys the mineral structure of gangue in uranium tailings by roasting pretreatment, making it loose and increasing pores, increase the specific surface area of the mineral, promote the full reaction of the mineral and the leaching agent, and improve the leaching efficiency. During the pyrolysis of uranium waste, a resistance furnace is commonly employed for thermal transfer. However, this method suffers from extended heating durations, elevated production expenses, substantial energy usage, and environmental contamination [
17,
18,
19]. Consequently, there is an urgent need to identify an alternative heating technique that is efficient, eco-friendly, and minimizes resource wastage.
Microwave is an electromagnetic wave with a frequency range between 300 MHz and 300 GHz, which makes polar molecules in the medium microwave field produce high-speed motion by the action of a high-frequency electric field, and the friction and collision between molecules generate heat [
20,
21]. It is different from the slow transfer of heat from outside to inside, microwave heating has the characteristics of volume heating, selective heating, fast heating, and easy control. Volume heating is reflected in the fact that the dielectric sample can be heated inside and outside simultaneously, thus greatly shortening the heat conduction time in conventional heating. In addition, due to the characteristics of volume heating, the temperature of the heated material is similar to the ambient temperature and the gas products produced by the chemical reaction of the molecules inside the material can escape through the surface. It will not seal the inside of the material due to surface melting or volume shrinkage [
22,
23,
24]. The selective heating is reflected in the fact that the ability of different non-metallic substances to absorb microwaves is strong and weak. Therefore, for the mixture, the components with strong microwave-absorption ability heat up quickly, and the substances with low absorption capacity heat up slowly. The microwave absorption capacity of a substance is predominantly governed by its dielectric loss factor, a parameter indicative of the material’s capacity to dissipate energy stored within it and transform it into thermal energy. In contrast to conventional thermal processing techniques, microwave heating facilitates selective material heating, thereby conferring several advantages, including localized thermal generation, accelerated thermal transfer rates, enhanced thermal efficiency, the stimulation of chemical reactions, and a lowering of the temperatures necessary for such reactions to occur. This targeted heating approach stands in stark contrast to traditional methods, offering a more precise and efficient alternative for material processing [
25,
26,
27,
28]. Lu et al. [
29] carried out research on microwave-assisted rock-breaking technology. The microwave electromagnetic differences of 11 kinds of diagenetic minerals were tested in a multimode cavity. It was found that the wave absorption of different mineral phases was different, and stress cracking occurred at lower temperatures. Amankwah et al. [
30] proposed a new process of microwave roasting pretreatment of gold ore containing silicate and other impurities. Making full use of the characteristics of microwave selective heating, thermal stress is generated inside the gold mine, thus destroying the mineral structure. After microwave roasting treatment, the mineral strength and cohesiveness have been effectively reduced. Olubambi et al. [
31] studied the mechanism of microwave pretreatment to improve the bioleaching behavior of low-grade complex sulfide ores. The number of cracks in the samples treated by microwave increased, the sulfur content decreased, and the pyrite phase increased. The change of mineral phase promoted the increase in solubility of microwave samples. The results show that microwave treatment improves the bioleaching behavior of the ore and greatly increases the leaching rate of copper and iron. Zhang et al. [
32] recovered aluminum from fly ash by microwave treatment. The results show that microwave heating technology is an effective and energy-saving method for the treatment of fly ash to recover aluminum. After microwave roasting at 800 °C for 60 s, 95% of the aluminum in fly ash was released. Liu et al. [
33] pretreated jarosite by microwave roasting technology, and recovered valuable metals by water leaching. Under the action of microwave heating, the elements in the mineral show a discrete distribution, which is conducive to the contact between the leaching solution and the mineral, and improves the leaching rate of valuable leaching. Zhang et al. [
34] used microwave roasting molybdenum concentrate combined with nitric acid leaching to prepare ammonium molybdate. Compared with conventional roasting, microwave roasting can greatly shorten the roasting time under the best conditions, and the obtained ammonium molybdate has higher purity and better stability. Li et al. [
35,
36] used microwave roasting technology to treat cyanide tailings. It was found that CaCl had a good ability to absorb microwaves. When the microwave power is 1300 W, the roasting temperature is set to 1137 °C, and the roasting time lasts for 15 min, the gold extraction rate reaches the best of 85.2%. Compared with conventional roasting, the extraction rate of gold has been significantly improved, and the energy consumption has been greatly reduced. Zeng et al. [
37] compared the conventional-roasting and microwave-roasting water-leaching process of red mud. The results show that under the same conditions, microwave roasting has a better alkali removal effect and lower power than conventional roasting. Microwave roasting can help to promote the crystal transformation of alkali components in red mud during the whole roasting process. Jena et al. [
38] successfully recovered potassium from unconventional silicate rocks by microwave-assisted roasting and subsequent water immersion. In the roasting process, charcoal is used as a microwave absorbing material to absorb microwave heating as a reaction heat source between CaCl
2 and nepheline synthetic rock. Aiming at the problems of low uranium-leaching rate and high energy consumption in the conventional-roasting nitric acid-leaching method, a microwave chlorination-roasting nitric acid-leaching method was proposed to improve the leaching rate and roasting efficiency of uranium and reduce energy consumption.
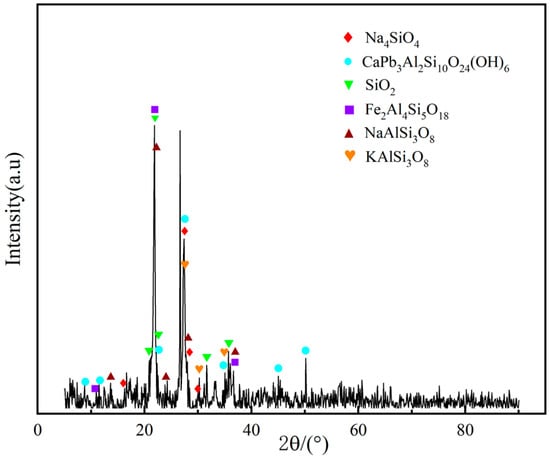
In this paper, NaCl is used as a chlorinating agent; NaCl as a medium-low-temperature chlorinating agent has the advantages of low price and low reaction temperature. The effects of microwave power, roasting temperature, NaCl addition, and roasting time on uranium leaching efficiency were studied. X-ray diffraction, differential thermal-thermogravimetric analysis, particle size analysis, scanning electron microscopy, and energy spectrum analysis were used to characterize the phase transition of tailings during microwave roasting, and the mechanism of sodium chloride destroying mineral structure during microwave roasting was explored. This was undertaken to achieve the purpose of efficient extraction of uranium from uranium tailings and also to provide technical support for the treatment of such radioactive solid waste.
Source link
Jinming Hu www.mdpi.com