1. Introduction
Bone defects caused by trauma, infection, or tumor resection present a significant clinical challenge, often requiring complex interventions to achieve effective bone regeneration [
1]. Annually, millions of patients worldwide undergo surgical procedures to address bone defects, leading to substantial social and economic burdens [
2]. Autologous bone grafts, considered to be the gold standard for bone regeneration, are limited by donor site morbidity and insufficient material availability [
3,
4,
5]. Allogeneic bone grafts, while a viable alternative, pose risks of immune rejection, disease transmission, and suboptimal integration with host tissues [
4,
6]. Synthetic bone substitutes, such as bone cements, are typically restricted to small, non-load-bearing defects due to their limited porosity and degradability, which are crucial for vascularization and long-term bone regeneration [
7,
8]. Therefore, the development of innovative therapeutic strategies that combine structural integrity, bioactivity, and antibacterial properties is urgently needed to overcome these limitations.
Tissue engineering has emerged as a promising strategy for bone defect repair, with scaffolds designed to mimic the natural bone microenvironment and promote regenerative processes. Scaffolds provide a three-dimensional (3D) architecture that supports cell adhesion, proliferation, and differentiation, while simultaneously delivering bioactive cues to enhance tissue regeneration [
9,
10,
11,
12]. Among the various scaffold fabrication techniques, 3D printing has gained significant attention due to its capacity to create complex, patient-specific structures with precise control over porosity, geometry, and material composition [
12,
13]. Polycaprolactone (PCL), a biodegradable polyester approved by the FDA for medical applications, is frequently employed in 3D printing owing to its favorable mechanical properties and processability [
14]. However, PCL lacks intrinsic bioactivity, necessitating the incorporation of bioactive components such as hydroxyapatite (HAp) and metal ions to enhance its osteogenic potential [
15].
To enhance the bioactivity of PCL scaffolds, HAp, a primary mineral component of natural bone, has been extensively utilized in bone tissue engineering due to its excellent biocompatibility and osteoconductive properties [
16]. The incorporation of HAp into PCL scaffolds has been shown to enhance osteogenic activity and mechanical strength, rendering it a suitable composite for bone regeneration [
15]. However, a critical limitation of HAp/PCL scaffolds is the absence of intrinsic antibacterial properties, which is a significant concern given the high risk of infection associated with bone defect repair, particularly in cases of trauma or complex fractures. Postoperative infections can severely compromise bone healing, leading to complications and implant failure [
17]. To address this limitation, researchers have explored incorporating metal ions, such as copper (Cu
2+), into scaffolds to impart both antibacterial and osteogenic functionalities [
18,
19,
20].
Copper, an essential trace element, plays a crucial role in numerous physiological processes, including angiogenesis, enzymatic activity, and bone metabolism [
21]. Recent studies have demonstrated that copper-doped scaffolds can enhance osteogenic differentiation and exhibit antibacterial activity, positioning them as promising candidates for bone defect repair [
19,
20]. However, uncontrolled copper release can induce cytotoxicity, highlighting the need for controlled release mechanisms to ensure therapeutic efficacy and safety [
22]. Metal–organic frameworks (MOFs), particularly copper-based MOFs (Cu-MOFs), offer a compelling strategy by enabling the sustained release of Cu
2+ while maintaining structural integrity and biocompatibility [
23,
24]. Cu-MOFs are porous crystalline materials composed of metal ions coordinated with organic ligands, providing a high surface area and tunable physicochemical properties [
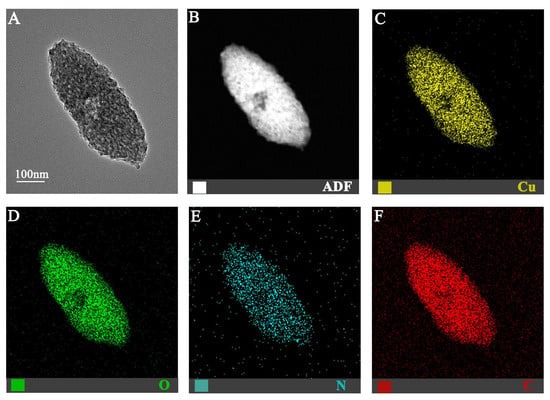
24]. Among Cu-MOFs, Cu-MOF-74 has attracted considerable interest due to its high density of open metal sites, which are known to enhance antibacterial and osteogenic properties [
25,
26]. The porous structure of Cu-MOF-74 facilitates controlled copper ion release, mitigating the risk of cytotoxicity associated with a burst release. Furthermore, the inherent properties of Cu-MOF-74 itself may contribute to enhanced biocompatibility and osteogenic potential within the scaffold. Given the critical challenge of bacterial infection in bone reconstruction—particularly in trauma settings, where compromised soft tissue and vascularization increase susceptibility to colonization [
27]—the incorporation of Cu-MOF-74 into bone scaffolds represents a rational approach to address both regenerative and antimicrobial requirements.
In this study, we developed Cu-MOF-74/HAp/PCL composite scaffolds using 3D printing. We hypothesized that incorporating Cu-MOF-74 into HAp/PCL scaffolds would enhance their antibacterial and osteogenic properties, rendering them suitable for bone defect repair and infection control. The scaffolds were characterized for their structural, mechanical, and biological properties, and their antibacterial and osteogenic potential was evaluated in vitro. Our findings demonstrate that Cu-MOF-74/HAp/PCL scaffolds exhibit excellent biocompatibility, antibacterial activity, and osteogenic potential, offering a promising solution for bone defect repair and regeneration.
4. Discussion
The development of multifunctional scaffolds that combine antibacterial and osteogenic properties represents a significant advancement in bone tissue engineering. In this study, we successfully fabricated Cu-MOF-74/HAp/PCL composite scaffolds using 3D printing technology and demonstrated their potential for bone defect repair and infection control. The incorporation of Cu-MOF-74 into HAp/PCL scaffolds not only enhanced their antibacterial properties but also promoted the osteogenic differentiation of bone marrow-derived mesenchymal stem cells (BMSCs), making them ideal candidates for clinical applications.
The structural characterization of the scaffolds revealed a uniform porous architecture with interconnected pores ranging from 100 to 200 μm, which is optimal for cell adhesion, nutrient diffusion, and vascularization [
28]. The addition of Cu-MOF-74 increased the hydrophilicity of the scaffolds, as evidenced by the reduction in water contact angles, a property known to enhance cell adhesion and proliferation [
29]. The sustained release of Cu
2+ ions from the scaffolds was observed over 28 days, with an initial burst release followed by a gradual release phase. This controlled release mechanism is critical for maintaining therapeutic copper concentrations while minimizing cytotoxicity [
23,
30]. The release kinetics of Cu
2+ was influenced by the degradation of the PCL matrix and the hydrolysis of Cu-MOF-74, which is consistent with previous studies on MOF-based drug delivery systems [
31].
The antibacterial properties of the scaffolds were evaluated against
S. aureus and
E. coli, two common pathogens associated with bone infections. The results demonstrated a concentration-dependent antibacterial effect, with the 1 Cu composite scaffolds showing a 90.07% reduction in
S. aureus and an 80.03% reduction in
E. coli. The antibacterial mechanism of Cu
2+ ions involves the disruption of bacterial cell membranes, generation of reactive oxygen species, and interference with DNA and protein functions [
32,
33]. These findings are consistent with previous studies demonstrating the potent antibacterial activity of copper-doped biomaterials [
19,
20,
34]. However, it is important to note that excessive copper release can lead to cytotoxicity, as observed in the 0.5% and 1% Cu-MOF-74 groups, which exhibited reduced cell viability and proliferation. Therefore, optimizing the concentration of Cu-MOF-74 is critical for balancing antibacterial activity and biocompatibility.
The osteogenic potential of the scaffolds was evaluated through ALP activity, calcium nodule formation, and the expression of osteogenic markers (BSP, OPN, and OCN). The 0.2% Cu-MOF-74 group exhibited the highest ALP activity and calcium deposition, indicating enhanced osteogenic differentiation of BMSCs. These findings are consistent with those of previous studies demonstrating the osteogenic effects of Cu
2+, which are known to promote angiogenesis and bone formation [
35,
36]. The upregulation of osteogenic markers, particularly BSP and OPN, further supports the role of Cu-MOF-74 in promoting bone regeneration. However, the 1% Cu-MOF-74 group showed reduced osteogenic activity, likely due to the cytotoxic effects of high copper concentrations. This highlights the importance of optimizing copper release to achieve therapeutic efficacy without compromising cell viability.
The multifunctionality of Cu-MOF-74/HAp/PCL scaffolds can be attributed to the synergistic effects of HAp and Cu-MOF-74. HAp provides a bioactive surface that promotes cell adhesion and osteogenic differentiation, while Cu-MOF-74 imparts antibacterial properties and enhances angiogenesis. These findings align with recent reports that Cu
2+ ions promote angiogenesis [
37,
38]. The combination of these components in a 3D-printed scaffold offers a promising solution for bone defect repair, particularly in infected or compromised bone sites. However, further studies are needed to evaluate the long-term in vivo performance of these scaffolds, including their degradation kinetics, mechanical stability, and ability to promote vascularized bone regeneration.
In conclusion, our study demonstrates the potential of Cu-MOF-74/HAp/PCL composite scaffolds for bone defect repair and infection control. Optimized concentrations of Cu-MOF-74 (0.05–0.2%) can effectively balance antimicrobial and osteogenic properties, presenting a promising strategy for clinical applications in bone defect repair. While these in vitro assays highlight the osteogenic potential of Cu-MOF-74/HAp/PCL scaffolds and their capacity to control infection and promote bone regeneration, further research is necessary to evaluate their long-term in vivo performance. Future studies should focus on assessing degradation kinetics, mechanical stability, and the ability of these scaffolds to promote vascularized bone regeneration in vivo, alongside continued optimization of Cu-MOF-74 concentrations and comprehensive evaluation in animal models.