1. Introduction
The seamless diving cylinders are known for their excellent low-temperature impact performance, lightweight design, and good corrosion resistance, making them widely applicable in diving activities [
1,
2]. Corrosion can weaken the strength and durability of these cylinders, making them more susceptible to physical damage such as cracking or deformation. This not only increases safety risks during use but may also lead to the premature retirement of the cylinder, resulting in higher replacement frequency and costs. Moreover, if the anti-corrosive layer on either the interior or exterior of the cylinder is compromised, gas may leak from damaged areas. This not only wastes valuable resources but could also pose potential hazards to both environmental health and human safety [
3]. Peeling paint or corrosion on the outer surface can significantly reduce the compressive strength, bending resistance of the wall, and overall corrosion resistance—factors that greatly affect the integrity of the cylinder. Such conditions can easily lead to internal explosions within the cylinder, posing serious risks to personal safety [
4]. Additionally, due to prolonged exposure in seawater environments, these alloy seamless cylinders are particularly prone to corrosive reactions which can substantially shorten the lifespan of critical components such as valves and regulators. The primary causes of corrosion in seamless breathing cylinders include seawater corrosion, crevice corrosion, and electrochemical corrosion. Materials exposed to marine environments may experience accelerated wear due to a synergistic effect between various forms of deterioration rather than solely from individual factors like simple corrosion or frictional wear [
5,
6,
7,
8].
The chromate passivation process exhibits excellent corrosion resistance and stable performance, having been applied in the industry for many years. However, due to the carcinogenicity, diffusivity, and bioaccumulation of Cr(VI), it poses significant threats to both environmental safety and human health. Therefore, developing high-corrosion-resistant chromium-free passivation processes is of great importance. The protective coatings prepared by the physical vapor deposition (PVD) technology exhibit excellent density and adhesion, which can coat the substrate surface with micro-level metal or non-metal compounds that are insoluble and exhibit outstanding corrosion resistance and wear resistance [
9,
10]. The composition and outcomes of these protective coatings have evolved from initial single-layer coatings to multi-layered, gradient-coated, nano-multilayer structures. Among these advancements, nano-multilayers have gained attention due to their superior wear resistance, strong adhesion between film and substrate, good corrosion resistance, high hardness, and long service life [
11]. In recent years, nano-composite TiBN coatings have demonstrated high hardness, excellent toughness, low friction, and good chemical stability, making them potential candidates for wear-resistant protective coatings [
12]. However, many of these reports lack depth in their investigation of the lubrication mechanisms associated with TiBN/base lubricating systems [
12]. They do not comprehensively consider the system design perspective, particularly regarding the mechanical properties of the films that significantly influence the solid–liquid composite system and their effects on friction and wear performance [
13]. In addition to these properties, TiBN coatings also exhibit significant advantages in marine corrosion resistance. The nano-hardness of TiBN coatings (ranging from 24 GPa to 34.5 GPa) strongly depends on the dual-phase structure of TiN/(amorphous) BN [
12]. At lower N
2 partial pressures, deposited TiBN coatings can achieve hardness values up to 40 GPa due to the formation of a metastable solid solution where B dissolves in face-centered cubic (FCC) TiN. Further increasing the N
2 partial pressure leads to the formation of a nano-composite material with a hardness of approximately 30 GPa composed of both TiN and BN [
13]. The addition of element B effectively reduces the grain size, significantly enhances the hardness of TiN coatings, and maintains good toughness, thereby avoiding the brittleness associated with BN and TiB
2 coatings.
Research indicates that ternary CrAlN coatings are particularly noteworthy because they possess high hardness, toughness, extended lifespan, strong abrasive wear resistance capabilities, as well as robust corrosion resistance and chemical stability [
14]. Additionally, studies suggest that Cr
2O
3 formed from chromium can serve a lubricating role by reducing friction [
15]. Adesina A.Y. et al. [
16] employed cathodic arc ion plating technology to deposit TiN, CrN, CrAlN, and TiAlN coatings on 304 stainless steel and investigated the corrosion behavior of these coatings in a corrosive medium consisting of 3.5% NaCl solution. The results indicated a significant positive shift in self-corrosion potential for all four coated samples compared to the 304 stainless steel substrate. Electrochemical impedance spectroscopy revealed that compared to similar nitrides formed via magnetron sputtering techniques, the deposited layers exhibited enhanced abilities against defect propagation and pore expansion when immersed in 3.5% NaCl solution. The addition of B elements in CrN-based coatings has become another focal point of research. However, single-layer coatings tend to grow continuously during the deposition process, which can lead to a columnar structure, allowing corrosive media to easily penetrate the coating material and even corrode the substrate, significantly reducing the corrosion resistance of the coating. To address this challenge, designing multilayer structures presents an effective method for enhancing both corrosion resistance and wear performance. Since the preparation process of multilayer coatings involves alternating deposition, differences in thermal expansion coefficients or lattice constants between different layers can result in dislocation stacking at the interfaces, which can hinder the formation of columnar structures within any given layer [
17]. Yu et al. [
18] utilized arc ion plating technology to fabricate CrYN/TiBN coatings and indicated that with increasing additions of Ti and B elements, both columnar growth and dense structures were eliminated, leading to a face-centered cubic CrN structure and amorphous BN structure being formed. The grain size was reduced from 33 nm to 15 nm, resulting in a denser coating that exhibited improved friction and wear performance.
Currently, there are relatively few research reports on CrAlN/TiBN nanocomposite multilayer coatings, and the investigation of their corrosion behavior in seawater environments remains to be explored. Therefore, this study employs arc ion plating technology to prepare TiBN, CrAlN, and CrAlN/TiBN multilayer coatings. A systematic examination is conducted on the evolution of the microstructural morphology of the coatings, their tribological properties, as well as their electrochemical corrosion behavior in artificial seawater and potential applications for protective measures in underwater gas cylinders.
4. Conclusions
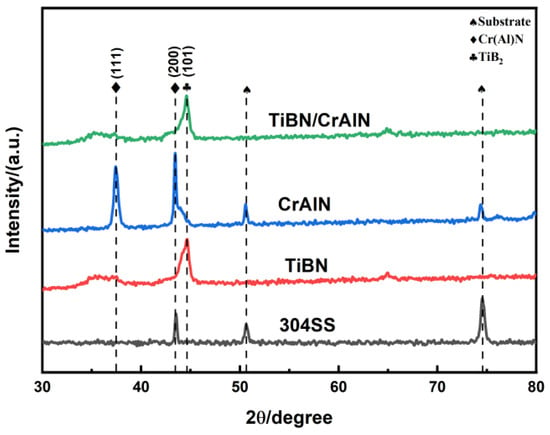
In the present study, we employed arc ion plating technology to deposit TiBN, CrAlN, and nano-multilayer of CrAlN/TiBN. The surface and cross-sectional morphology, tribological performance, and electrochemical corrosion resistance of the three coatings were investigated. The results indicate that the preferred orientation of the TiBN coating is TiB2 (101), while for the CrAlN coating, it is Cr(Al)N (200). In contrast, the preferred orientation for the CrAlN/TiBN multilayer coating is also TiB2 (101). The multilayer structure refines grain size and disrupts columnar grain growth, resulting in a denser microstructure at the cross-section of these coatings. Tribological testing demonstrated that the friction coefficient follows this order: CrAlN/TiBN (0.489) > CrAlN(0.491) > TiBN (0.642), with CrAlN/TiBN exhibiting the lowest friction coefficient. Electrochemical tests conducted in artificial seawater showed that among these coatings, CrAlN/TiBN had the highest self-corrosion potential, followed by CrAlN. From the Bode plots analysis, it can be concluded thatCrAlN/TiBN displayed a maximum phase angle compared to other coatings. Due to its superior corrosion resistance properties, CrAlN/TiBN holds significant application value for protective measures on underwater breathing gas cylinders. The subsequent research will focus on the fatigue characteristics of coatings and the interactions between corrosion and friction. Additionally, a more diverse range of substrates, such as AISI 316, aluminum alloys, and carbon steel, will be used.