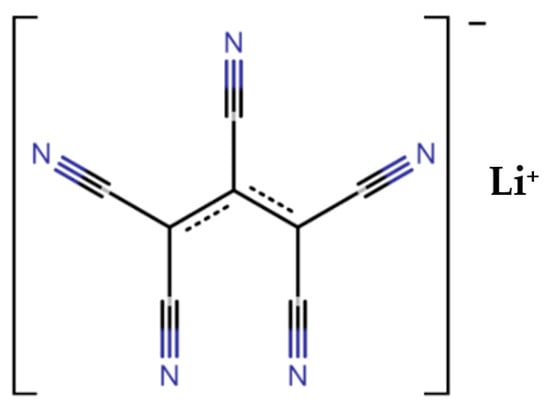
1. Introduction
Therefore, in this work, we report the viability of this new fluorine-free lithium salt, LiPCP with fluorine-free electrolyte additives working with aqueous-processed LFP and LMFP cathodes. For this purpose, we report the electrolyte conductivity at different concentrations and temperatures, an analysis of the electrolyte stability, and an analysis covering voltammetry and galvanostatic cycling, demonstrating the capability of fluorine-free electrolytes for aqueous-processed olivine-type cathode materials. These findings represent the initial phase of investigating safer and more environmentally friendly electrolytes with aqueous-processed electrodes for lithium-ion batteries.
3. Materials and Methods
3.1. Reagents
Ethylene carbonate (EC), dimethyl carbonate (DMC), ethyl-methyl carbonate (EMC), diethyl carbonate (DEC), and vinylene carbonate (VC) battery-grade solvents were purchased from BASF (Ludwigshafen am Rhein, Germany). Lithium hexafluorophosphate (LiPF6, battery grade) and acetonitrile (AN, anhydrous, 99.98%) were obtained from Sigma Aldrich (Saint Luis, MO, USA). A total of 1 mol·kg−1 LiPF6 in EC:DMC (30:70 wt.%) was purchased from E-Lyte Innovations (battery grade). The metallic lithium foil was purchased from Honjo Metal. The lithium iron phosphate (LFP, LiFePO4, 1.45 wt.% carbon) and lithium iron manganese phosphate (LMFP, LiMn0.6Fe0.4PO4, 1.5~2.5 wt.% carbon) powdered materials were acquired from MTI (Richmond, CA, USA) and MSE Supplies, respectively. Sodium carboxymethyl cellulose (CMC, average Mw of 250,000, degree of substitution equal to 0.9) was purchased from Sigma Aldrich. Super-P and Ketjenblack conductive carbons were procured from Alfa Aesar and MSE Supplies, respectively. The carbon-coated aluminum foil (15 µm thickness of aluminum, 1 μm of conductive carbon) was purchased from MSE Supplies.
3.2. Electrolyte Preparation
The fluorine-free electrolyte solutions were prepared in an argon-filled glovebox (LabStar, MBraun, H2O < 1 and O2 < 10 ppm, Garching, Germany)) by dissolving the LiPCP salt in different solvents. For solvent screening, electrolyte solutions of 0.8 mol·kg−1 LiPCP in EC:DMC (30:70 wt.%), EC:EMC (30:70 wt.%), and EC:DEC (30:70 wt.%) were prepared. For comparison, the commercial standard 1 mol·kg−1 LiPF6 electrolyte solutions were prepared in the same solvent mixtures. Additionally, for molality screening, solutions from 0.1 to 1.2 mol·kg−1 LiPCP in EC:DMC (30:70 wt.%) were prepared.
3.3. Ionic Conductivity Measurements
The ionic conductivity of the LiPCP-based electrolytes was obtained via electrochemical impedance spectroscopy (EIS) using a VMP3 potentiostat-galvanostat (VMP3, Bio-Logic, Seyssinet-Pariset, France) in the temperature range of 0 °C to 50°C. The measurements were carried out in the frequency range of 500 kHz to 1 Hz with 10 points per decade and a signal amplitude of 5 mV. The measurement at each frequency was repeated 6 times. The conductivity micro cells consisted of an electrolyte placed between two stainless steel electrodes. A cryostat-thermostat (Haake K75), (Vreden, Germany) with a temperature controller (DC50) was used for the thermoset samples.
where k is the cell constant (0.3–0.7 cm−1, determined with ± 0.3% precision) for each conductivity microcell and R is the bulk resistance in ohms (Ω) obtained from the respective Nyquist plot.
3.4. Linear Sweep Voltammetry
Linear sweep voltammetry (LSV) was used to evaluate the electrochemical stability window of the following electrolytes: 0.8 mol·kg−1 LiPCP in EC:DMC (30:70 wt.%), 0.8 mol·kg−1 LiPCP in EC:DMC (30:70 wt.%) + 5 wt.% VC, 0.8 mol·kg−1 LiPCP in EC:DMC (30:70 wt.%) + 5 wt.% VC + 5 wt.% AN, and 0.8 mol·kg−1 LiPCP in EC:DMC (30:70 wt.%) + 5 wt.% VC + 10 wt.% AN. The tests were conducted at room temperature in a two-electrode Swagelok-type cell configuration at a scan rate of 0.5 mV s−1. For the set-up, a Pt disc was used as the working electrode and a Li metal disc as the counter and reference electrode.
3.5. Lithium Passivation Measurement
Lithium passivation was investigated with electrochemical impedance spectroscopy (EIS) for the following electrolytes: 0.8 mol·kg−1 LiPCP in EC:DMC (30:70 wt.%) + 5 wt.% VC, 0.8 mol·kg−1 LiPCP in EC:DMC (30:70 wt.%) + 5 wt.% VC + 10 wt.% AN, and 1.0 mol·kg−1 LiPF6 in EC:DMC (30:70 wt.%). The measurements were carried out in the Swagelok-type cells with two lithium electrodes (discs) with a Celgard 2400 separator in between of them. For the measurement, a VMP3 potentiostat-galvanostat (VMP3, Bio-Logic) was used. The measurements were carried out in the frequency range of 500 kHz to 1 Hz, with 10 points per decade and signal amplitude of 5 mV. The measurement at each frequency was repeated 6 times. The impedance spectra were repeatedly measured in a period of 6 h. The Impedance spectra were analyzed using RelaxIS 3 software.
3.6. Electrode Preparation
LFP and LMFP were used independently as the active materials (AM). CMC was employed as the binder. Super-P and Ketjenblack conductive carbon black were used independently as the conductive additives (CMs) for electrode preparation. The components were mixed following different AM:CM:binder ratios (as indicated where appropriate throughout the manuscript) by using magnetic stirring (IKA-type RH Basic Magnetic Stirrer, Staufen im Breisgau, Germany) at 500 rpm for 24 h, and distilled water as the solvent. The obtained slurry was then cast onto carbon-coated aluminum foil by using a blade coater (Doctor Blade, ZEHNTNER Testing Instruments, ZAA 2300, and ZEHNTNER film applicator ZUA 2000, Sissach, Switzerland). All electrodes were first dried at 80 °C for 2 h, then, subsequently, they were vacuum dried at 120 °C overnight (Memmert VO 400, Schwabach, Germany)).
3.7. Cyclic Voltammetry Measurement
To evaluate the electrode/electrolyte compatibility, cyclic voltammetry (CV) measurements were conducted in a potentiostat-galvanostat (VMP3, Bio-Logic). LFP and LMFP electrodes of 11 mm in diameter were punched and assembled in two-electrode Swagelok-type cell configuration using a disc of Li metal as a counter electrode. Celgard 2400 microporous polypropylene was used as the separator, 0.8 mol·kg−1 LiPCP in EC:DMC (30:70 wt.%) was used as the electrolyte, and 1 mol·kg−1 LiPF6 in EC:DMC (30:70 wt.%) was used as the reference electrolyte. The scan rate was set at 0.5 mV·s−1 in 2.5–4.0 V vs. Li+/Li potential limits for the LFP cathode and 2.5–4.5 V vs. Li+/Li potential limits for the LMFP cathode.
3.8. Galvanostatic Cycling
For galvanostatic cycling, LFP and LMFP electrodes of 15 mm in diameter were punched and assembled in CR2032 coin cells, using a Li metal disc as the negative electrode and Celgard 2400 as the separator. The electrolyte solution was 0.8 mol·kg−1 LiPCP in EC:DMC (30:70 wt.%) + 5 wt.% VC. The tests were performed in a SOLLICH potentiostat-galvanostat (SOLLICH 2061 MPG&T, Multichannel Potentiostat-Galvanostat and Battery Tester) in 2.5–3.9 V vs. Li+/Li potential limits for the LFP cathode and 2.4–4.2 V vs. Li+/Li for the LMFP cathode. The protocol consisted of one formation cycle at C/25 (where C = 170 mAh·g−1 in the case of LFP and 160 mAh·g−1 in the case of LMFP) followed by C/10 cycling. In addition, the rate capability of the LFP and LMFP cathodes with the LiPCP-based electrolyte was screened. To this purpose, 1 cycle at C/25, 5 cycles at C/20, 5 cycles at C/10, 5 cycles at C/5, 5 cycles at C/2, and 5 cycles at 1C were performed.
4. Conclusions
In summary, the electrochemical performance of aqueous-processed olivine-type LFP and LMFP electrodes was systematically investigated using a fluorine-free LiPCP-based electrolyte. The precise optimization of the electrolyte composition revealed that an EC:DMC (30:70 wt.%) mixture provided the highest ionic conductivity. The optimal concentration of LiPCP was found to be 0.8 mol·kg−1, achieving a conductivity of 9.6 mS·cm−1 at 20 °C. This electrolyte exhibited electrochemical stability up to 4.4 V vs. Li+/Li when VC was utilized as a solid electrolyte interphase (SEI)-stabilizing additive, resulting in a fully fluorine-free electrolyte with favorable characteristics.
A comprehensive screening of various compositions for aqueous-processed LFP and LMFP electrodes was conducted. The optimal component ratio was found to be 87:10:3 (AM:CM:binder). Two conductive additives, Super P and Ketjenblack, were evaluated, with Ketjenblack consistently delivering a superior electrochemical performance. The concentration of CMC in the binder solution during electrode preparation was varied, and a concentration of 1% yielded the most favorable electrode properties. The selected electrode formulation proved suitable, achieving specific capacities of 150 mAh·g−1 (LFP) and 125 mAh·g−1 (LMFP) with the reference LiPF6 electrolyte.
LiPCP-based electrolytes, incorporating fluorine-free linear carbonates as solvents and a stabilizing additive, exhibited fast kinetics and stable reversible cycling. Stable galvanostatic cycling of half-cells confirmed the compatibility between the LiPCP and olivine-type cathodes. The electrochemical performance of LFP with LiPCP was comparable to that with LiPF6. On the contrary, the performance of LMFP with LiPCP was inferior, attributed to increased cell polarization, which hindered the full utilization of the high-potential plateau of this cathode. While LiPCP is compatible with LMFP, further optimization of the electrolyte composition for this system is necessary.
In conclusion, lithium-ion batteries devoid of critical and problematic elements such as cobalt, nickel, and fluorine were engineered and exhibited a satisfactory performance. These findings represent the initial phase of a groundbreaking investigation into developing safer and more environmentally friendly electrolytes compatible with aqueous-processed electrodes for lithium-ion batteries. This first stage lays the foundation for a novel approach, aiming to innovate the battery industry by reducing its dependence on hazardous materials and assisting in improving the overall sustainability and safety of energy storage systems.
Source link
Claudia Limachi www.mdpi.com