1. Introduction
The capitulum, a distinctive head-like inflorescence, is considered a pivotal morphological innovation for the evolutionary success of Asteraceae [1]. The inflorescence is composed of multiple florets on a single receptacle and surrounded by involucral bracts. These florets are usually divided into ray and disc florets, which are monosymmetric female (or sterile) and polysymmetric bisexual flowers, respectively [2]. The sophisticated and fascinating inflorescence architecture has attracted the attention of many botanists.
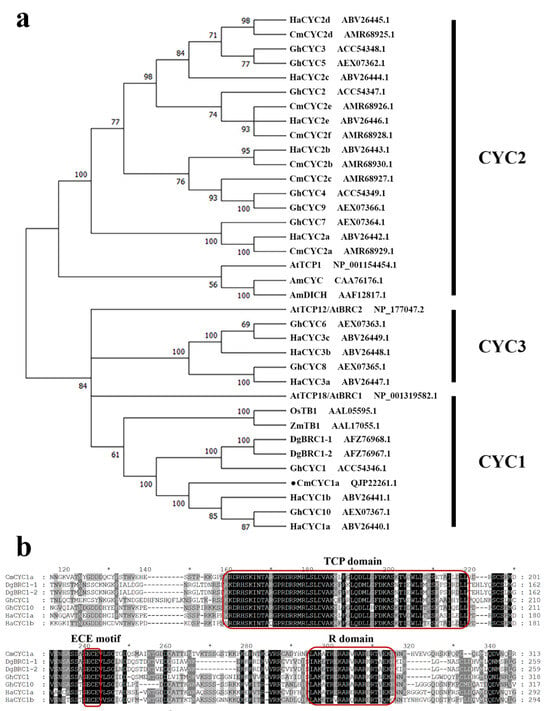
The ECE clade, known as the CYCLOIDEA (CYC)/TEOSINTE BRANCHED 1 (TB1) clade of the TCP family, primarily participates in the formation of flowers or branches from axillary meristems [3]. Phylogenetic analysis suggests that the ECE clade has experienced two rounds of gene duplication within core eudicots, resulting in three subclades: CYC1, CYC2, and CYC3 [4]. Among them, the key role of the CYC2 subclade in regulating flower symmetry has been a hot issue since the CYC gene was first characterized in Antirrhinum majus [5]. CYC and its paralog DICHOTOM (DICH) are continually expressed in dorsal regions of floral meristems and dorsal primordia at later stages, affecting flower symmetry, petal size, and petal shape and forming a staminode [6,7]. In Helianthus, Gerbera, Senecio, Chrysanthemum, and other Asteraceae groups, CYC2 genes are mainly expressed in ray florets, with low or no expression in disc florets. Functional analyses also indicated their independent recruitment in differentiating floret identity [8,9,10,11,12,13,14,15].
Arabidopsis TCP18/BRANCHED 1 (BRC1) is a CYC1 gene closely related to maize TB1, which suppresses axillary organ growth and generates female inflorescences [16]. In Arabidopsis thaliana, BRC1 promotes the growth arrest of axillary buds and interacts with FT and TSF proteins to prevent floral transition [17]. Unfortunately, although BRC1 and TCP12/BRC2, CYC3 genes, are both expressed in the provascular tissue of flowering buds, their functions during flower development remain unclear [18]. In Petunia axillaris, three CYC1 genes (PaTCP18a/b/c) are expressed in inflorescences and flower buds, and they are differentially expressed in the petals of two selfing lines with small and large flowers, suggesting a possible role in flower development and petal growth [19]. However, up to now, information on the function of CYC1 genes in floral development has been quite limited.
Throughout the angiosperms, ECE genes have experienced multiple independent and lineage-specific duplications [20]. The duplication of CYC1 genes may provide the basis for the change in flowers from solitary to complicated inflorescences (including the capitulum) in Campanuloideae [21]. It is also hypothesized that the two plant groups with a capitulum, Dipsacaceae and Asteraceae, may have taken advantage of ECE gene duplication [2,22,23]. In Dipsacaceae, radiate species bearing both ray and disc florets have more than two times the number of ECE genes than discoid species, which contain only disc florets [23]. In Anacyclus clavatus [24], C. morifolium [25], Helianthus annuus [8], and Gerbera hybrida [11] (Asteraceae), ECE genes have also undergone several rounds of gene duplication, all resulting in 10 ECE members. Furthermore, genome-wide analyses have identified 13 ECE members in C. lavandulifolium [26]. GhCYC10 and HaCYC1a, two CYC1 genes, are highly expressed in ovary tissue, as are CYC2 genes. Notably, in contrast to CYC2 genes, HaCYC1a and ClCYC1a are expressed at higher levels in disc florets than in ray florets [11]. In Knautia macedonica (Dipsacaceae), KmCYC1 is significantly differentially expressed in the ventral, lateral, and dorsal petals of external florets and in the ventral petals of external and internal florets, as is KmCYC2, indicating a potential role in determining flower symmetry [27]. Moreover, yeast two-hybrid (Y2H) assays performed in gerbera (G. hybrida) and sunflower (H. annuus) have identified interactions between the proteins belonging to the three different subclades of ECE [11]. Therefore, CYC1, CYC2, and CYC3 subclades may be functionally related, and CYC1 and CYC3 genes may also participate in the formation of a head-like inflorescence.
C. morifolium, a typical example used to explore flower symmetry in Asteraceae, has a rich variety of flower types and has been widely planted around the world. However, the detailed genetic mechanism regulating the complex chrysanthemum inflorescence still remains unknown [28]. Our previous studies have identified six CYC2 genes and confirmed the interactions between CYC2 transcription factors in regulating flower symmetry [14,29]. We wondered if CYC1 genes play any role in chrysanthemum flower development and whether they are in any way related to CYC2 genes. RNA-Seq analysis of the chrysanthemum cultivar ‘Jinba’ has identified two CYC1 genes and they were highly expressed in immature inflorescences [25]. Chen et al. have reported a CYC1 gene, DgBRC1, from ‘Jinba’ and found it to be functionally conserved in regulating lateral branching [30]. However, the function of DgBRC1 in flower development was not mentioned. In this study, we isolated another CYC1 gene, CmCYC1a, and explored its expression patterns during inflorescence development. Overexpression of CmCYC1a in A. thaliana significantly altered flower symmetry and the number of stamens. Additionally, Y2H assays were performed to investigate pairwise protein–protein interactions between CmCYC1a and CmCYC2. Our results provide a candidate gene, CmCYC1a, for regulating flower symmetry and stamen development which may act together with CmCYC2 in the formation of the chrysanthemum capitulum. This study is an essential step toward deciphering the involvement of three subclades of ECE genes in determining the morphogenesis of the capitulum.
4. Discussion
Prior studies of the CYC1-like ortholog ZmTB1 in maize found that ZmTB1 controls apical dominance, arrests stamen growth, and generates female inflorescences [16]. The function of CYC1-like orthologs in preventing branch outgrowth has been validated in many other species, including both monocots and dicots, such as O. sativa (OsTB1) [46], A. thaliana (AtBRC1/AtTCP18) [18], Solanum lycopersicum (SlBRC1) [47], C. morifolium (DgBRC1) [30], and Medicago truncatula (MtTCP18) [48]. However, few studies have reported the role of CYC1 genes in floral development. In Papaveraceae, two PAPACYL paralogs, closest to AtBRC1/AtTCP18, are expressed during flower development in three species that differ in flower symmetry. Interestingly, PAPACYL transcripts are asymmetrically expressed in the outer petals of the monosymmetric species and expressed in the developing anther of the bisymmetric species, suggesting a correlation with flower symmetry and stamen development [49]. In K. macedonica, KmCYC1 is expressed significantly more in the lateral and dorsal petals compared with the ventral petals of external florets, indicating a possible association with flower symmetry [27]. Transcriptome analysis of the chrysanthemum cultivar ‘Jinba’ has identified two CYC1 genes and they were both highly expressed in immature inflorescences, suggesting their involvement in inflorescence formation [25].
In this study, a CYC1 gene CmCYC1a was isolated in the chrysanthemum cultivar ‘Fen Ditan’ and the expression level of CmCYC1a was increased successively during the flowering process in both disc and ray florets, suggesting that CmCYC1a is associated with the growth of the two florets or the chrysanthemum capitulum. Additionally, CmCYC1a was expressed at higher levels in disc florets than in ray florets, consistent with the sunflower HaCYC1a. Actually, in both gerbera and sunflower, HaCYC1a is the only CYC1 gene with differential expression between different flower types [11], indicating the involvement of CmCYC1a and HaCYC1a in the differentiation of flower types. Floral tissue-specific expression analysis at later stages of flowering found that CmCYC1a was expressed in all of the tissues examined, suggesting its extensive involvement in chrysanthemum flower growth and development.
To further explore the functions of CmCYC1a, the gene was overexpressed in Arabidopsis. In 35S::CmCYC1a lines, the flowers were altered from radially symmetrical to bilaterally symmetrical with fewer stamens. In Arabidopsis with ectopic expression of three Canna indica ECE genes, a similar phenotype was reported. Moreover, the altered flower symmetry of transgenic Arabidopsis is thought to be caused by the loss of stamens [50]. On the other hand, our previous study found that overexpression of CmCYC2 in the tcp1 mutant also changed flower symmetry but not the number of stamens [29]. And overexpression of two transcripts of Cyc2CL, a CYC2 gene cloned from ‘Fen Ditan’, resulted in the inhibition of petal and stamen development in Arabidopsis [35]. Since the ancient paralogues may stay in the same regulatory network [51], our results indicate that CmCYC1a may have retained the CYC1-like gene function in suppressing stamen growth and is functionally redundant along with CmCYC2 in regulating floral symmetry and stamen development. Indeed, it has been proposed that TCP1 may act redundantly along with BRC1/2 in leaf development [52]. The redundant functions of gerbera CYC2 genes GhCYC2/3/4 have been demonstrated, and their ectopic expression in gerbera disc florets resulted in transition to ray-like florets and the inhibition of stamen development [12]. Therefore, our speculations can be further verified in chrysanthemum with CRISPR/Cas9 [53] or other plant transformation methods [54].
Protein–protein interactions showed a complex network involving CYC1 and CYC2 proteins in chrysanthemum. Our previous study has confirmed that CmCYC2b can form heterodimers with CmCYC2d and CmCYC2e, and CmCYC2c can form a heterodimer with CmCYC2d [29]. Here, CmCYC1a-CmCYC2b, CmCYC1a-CmCYC2d, and CmCYC1a-CmCYC2f were revealed in the Y2H assays, suggesting that CmCYC2b and CmCYC2d may be key factors in the network linking the two subclades, and CmCYC1a and CmCYC2 may participate together in capitulum morphogenesis through protein interactions. Notably, the interactions observed in the Y2H assays can be further validated by other experiments such as bimolecular fluorescence complementation (BiFC) [55] and surface plasmon resonance (SPR) [56]. In addition, overexpression of CmWUS, a WUSHCHEL-like gene, also affects flower symmetry and inhibits stamen and pistil development in Arabidopsis, and CmWUS can interact with CmCYC2d [33]. The Y2H assays performed in C. lavandulifolium have found that A-class MADs-box proteins DenFUL and ClAP2 can form heterodimers with ClCYC2d and ClCYC2e [57]. In gerbera, the C and E class protein pair GAGA1-GRCD5 may suppress the expression of GhCYC3, which specifies ray floret identity, and affect stamen development [58]. Furthermore, the negative feedback loop between WUS and AGAMOUS (AG), a C class gene, has been established in Arabidopsis [59,60]. Hence, a more complex molecular regulatory network of floral development in chrysanthemum encompassing ECE, WUS, and MADS-box has emerged and is waiting to be explored.
In particular, CmCYC1a was highly expressed in involucral bracts and receptacles, as was the previously reported Cyc2CL-1 in ‘Fen Ditan’ [35]. CmCYC2 and Gerbera ECE genes, like GhCYC2, GhCYC9, and GhCYC10, are also highly expressed in involucral bracts [11]. In C. lavandulifolium, ClSVPa, a MIKCc-type MADS-box gene, is specifically expressed in involucral bracts and receptacles [61]. The protein–protein interaction between ClCYC2e and DenFUL is thought to have a possible role in receptacle identity [57]. In some T1 plants of 35S::CmCYC1a, the flowers showed strong phenotypes (Figure S3) with disrupted floral organs, including abnormal sepals, similar with Arabidopsis overexpressing Cyc2CL [35] or GhCYC4/7 [12] and SVP mutants in the CRISPR/Cas9 system [62], indicating other functions of CmCYC1a in floral development. But due to floral abortion, these T1 plants could not be screened and observed afterwards. Thus, the specific role of CmCYC1a in the development of involucral bracts and receptacles and its relationship with CYC2 and MADS-box genes are unclear and await more research for elucidation.
TCP transcription factors are mediators of hormone activity and key players in hormone signaling [63]. In the promoter region of C. lavandulifolium TCP genes, many hormone response elements were found [26]. The critical role of auxin concentration in determining floret identity has already been reported in the studies of Senecio vulgaris and Matricaria inodora. Additionally, the expression of MiRAY2, a CYC2 gene, is affected by auxin [64]. Integrated transcriptomics and metabolomics analyses also revealed a significant correlation between auxin and genes related to flower development, such as TCP and MADs-box genes, in Argyranthemum frutescens (Asteraceae) [65]. The expression of the CYC1 gene BRC1 is influenced by diverse phytohormones, including auxin, cytokinins, strigolactones, and gibberellin, but the detailed mechanism is still confusing [66]. In chrysanthemum, the transcript level of DgBRC1 is also associated with auxin and cytokinin [30,67]. We propose that CmCYC1a may be involved in hormonal signaling networks, especially in relation to auxin, during flower development, but this speculation needs to be further verified.
Source link
Yi Yang www.mdpi.com