1. Introduction
The joining of dissimilar materials is very attractive and useful currently, following the ever increasing standards for more efficient manufacturing processes in industrial fields [
1,
2]. By combining materials with differing thermophysical properties, it is possible to obtain a component possessing the advantages of each of the two materials. Copper and aluminum are highly sought-after materials due to their excellent malleability, electrical and thermal conductivity, high strength-to-weight ratio, and other qualities [
3,
4]. However, the joining of the two materials is highly limited due to the very low solubility of copper in aluminum, with a maximum value of about 5.65–5.80% [
5]. Once oversaturated, the aluminum solid solution releases Cu atoms, which form bonds with the aluminum particles in the form of intermetallic compounds (IMCs) of the Cu
xAl
x type [
6]. The most commonly formed IMC is CuAl
2, which is known to be brittle [
7]. The presence of IMCs in joints between Cu and Al are a prerequisite for weakening the structure and premature fracture.
The most common method used for the joining Cu and Al is friction stir welding due to the very controlled process of mixing the two materials, which results in a reduced amount of formation of IMCs [
8,
9]. However, a highly desirable method that can potentially be used for this process is electron beam welding. Its main advantages are high precision, excellent controllability and potential for automation, high purity of the formed weld due to the absence of atmospheric gasses, the high penetration of the electron beam that makes it ideal for welding of butt joints, and so on [
10]. A major issue is present with this process, however, which is related to the accelerated formation of IMCs. A well-known strategy used to limit the amount of IMCs formed is the reduction in Cu inclusion in the weld seam by offsetting the electron beam towards the aluminum component, which is also used in laser welding techniques [
11]. Applying an offset towards the aluminum’s surface, however, creates another problem related to the rapid over-melting of the aluminum component, which occurs due to the low melting and boiling temperatures of Al. To resolve this issue, a circular beam oscillation can be used, as shown by [
12]. Another method for the limitation of formation of IMCs is the application of a filler between the welding surfaces, which limits the incorporation of Cu in the melt pool, and thus the formation of the undesirable IMCs [
13].
Regardless of the current advances in electron beam joining of Cu and Al, much more data need to be collected in order to determine the optimal technological conditions required for this operation. Due to this, in the present work, the power of the electron beam was varied in order to observe its influence on the mechanisms of formation of intermetallic compounds during electron beam welding. The structure of the weld seams and their microhardness and tensile strength were also investigated. The results are discussed for the benefit of possible future improvements in the process.
2. Materials and Methods
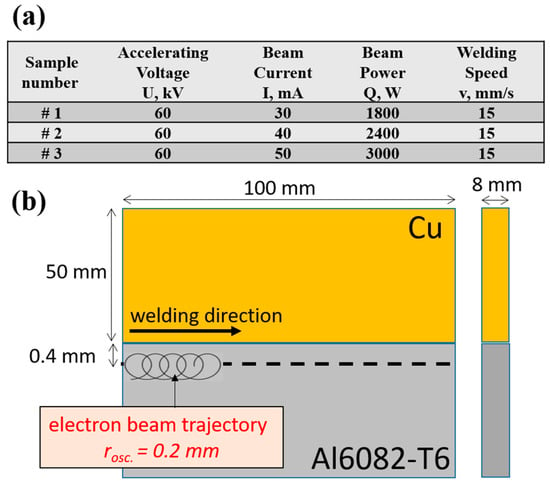
The metal plates used in the present work have a size of 100 × 50 × 8 mm and are made of Cu (99.8%) and an Al6082-T6 alloy. The chemical composition of the Al6082-T6 plate is as follows (wt%): 98.16% Al, 1.15% Mg, 0.32% Si, and 0.36% Mn. The plates were cut and the working surface areas were milled and ground with abrasive paper in order to reduce the distance between the plates during the welding process as much as possible. The schematic of electron beam welding with an offset and an oscillation of the electron beam is shown in
Figure 1b. The electron beam welding was carried out on Evobeam cube 400, Evobeam GmbH, Nieder–Olm, Germany welding machine. Some of the technological conditions are presented in the table shown in
Figure 1a. Prior to the welding procedure, both plates were cleaned with acetone and preheated to a temperature of 300 °C in an external furnace. The preheating process was performed in order to reduce the thermal internal stresses caused by the high thermal gradient formed during the EBW process. The temperature used in this work was chosen so that it was specifically lower than the temperature of oxidation of copper in air [
14]. It is important to note that the reached maximum temperature of 300 °C was definitely reduced to an extent, influenced by the high thermal conductivity of copper and aluminum and also by the vacuum chamber depressurization time (about 30 s). The welding procedure was carried out using a welding speed of 15 mm/s, a circular oscillation with a radius of 0.2 mm and a frequency of 200 Hz, and an offset of 0.4 mm of the electron beam on the aluminum plate side. The accelerating voltage was constant, with a value of 60 kV. The welding current was varied, with values of 30 mA, 40 mA, and 50 mA, totaling the power of the electron beam to 1800 W, 2400 W, and 3000 W. The specimens were evacuated from the vacuum chamber 20 min after the process, after which they were placed in a furnace (TR 240, Nabertherm GmbH, Lilienthal, Germany) at a temperature of about 100–120 °C, where they were gradually cooled down to room temperature over the course of 60 min. A 2 °C per minute cooling process was determined to be sufficient enough to avoid the formation of cracks in the weld seam caused by rapid cooling. It is also important to note that although different cooling rates could also relieve the stresses during the cooling process successfully, the most favorable possible case was chosen in the current research in order to guarantee the successful evasion of crack propagation along the length of the weld seam.
Metallographic samples of the cross-section of the formed welds and the raw materials were prepared using a sequence of cutting, grounding, and etching. The abrasive paper strategy used was as follows: grit 240, 400, 600, 800, 1200, 2500, and 4000. A combined etching method was used in order to etch both materials using a 10% solution of HF acid for the aluminum side of the sample, and an FeCl3 + HCl solution for the copper side of the sample. The optical images obtained in this work were taken using a Drawell MIT 300/500 microscope (Drawell Instrument Co., Ltd., Chongqing, China). Additional optical images of the untreated Al6082-T6 alloy and the heat-influenced alloy were taken using the same microscope. The same polishing steps were used as described above; however, in this case, the samples were etched using Tucker’s reagent. It comprised 25 mL dH2O, 15 mL HF, 25 mL HCl, and 15 mL HNO3. The samples were swabbed for 10 s each.
To perform a phase analysis of the resulting welded joints, an X-ray diffractometer (XRD), “Bruker D8 Advance” (Bruker, MA, USA), was used. The used method was “Two theta” (Bragg–Brentano). The characteristic X-ray radiation was CuKα, with a wavelength of 1.54 Å. A step of 0.1 degrees and a registration time of 0.5 s per step was applied. All measurements were performed in an area close towards the center of the weld seam. The X-ray beam had a circular shape with a diameter of 1 mm (thus having a scanning area of 0.785 mm
2), as shown in
Figure 2.
The structures of the welded samples were investigated using a scanning electron microscope (SEM), “LYRA I XMU” (TESCAN, Brno, Czech Republic), in the back-scattered electrons mode, equipped with an energy dispersive X-ray spectroscopy (EDS) “Quantax 200” (Bruker, MA, USA) unit.
The microhardness experiment was performed on a semi-automatic microhardness tester, “ZWICK/Indentec—ZHVμ-S” (ZwickRoell, Ulm, Germany). A load force of 0.5 N was used for all experiments. The microhardness of the weld joints was measured following a linear pattern, starting from the copper side of the samples, moving through the fusion zone, and ending on the aluminum side of the samples. The measurements were performed at a depth of the cross-section of 2 mm below the surface of the samples.
A ZwickRoell Vibrophore 100 unit (ZwickRoell, Ulm, Germany) was used in order to determine the tensile properties of the samples. A static strain method was applied with a strain rate of 30 MPa/s. The dimensions and a visual representation of the tensile test samples are shown in
Figure 3. The length of the test zone was 40 mm, and the surface area was 320 mm
2.
4. Discussion
During the welding process, a number of physical phenomena are present, such as melting of the solid state materials, formation of defects during the liquid stage, cooling processes, followed by solidification of the melt pool, formation and rearrangement of solid state crystals, migration of defects during the recombination processes, potential plastic deformation, and more. Each of these processes can have a major effect on the output structure of the weld seam. During the solidification stage, the priority of the molten phase is to crystallize with minimal internal strains (achieving the highest possible equilibrium). This process is problematic even when welding similar materials, particularly those that change their phase composition due to the high temperature (such as steel, titanium and others). Welding aluminum, on the other hand, leads to the formation of defects and their migration such that clusters of vacancies form. This causes the separation of some of the aluminum crystals and the formation of hollow cavities (pores). This type of defect is commonly referred to in the literature as a solidification pore [
20]. It is obvious from the SEM analysis that a number of solidification pores are present in both the first and second samples formed with a beam power of 1800 W and 2400 W. No such defects are observed in the case of the last sample formed with a power of 3000 W. Instead, a clearly defined secondary phase is observed, mostly consisting of the CuAl
2 phase, as suggested by both the XRD and SEM analysis. It is obvious from the SEM images that a highly inhomogeneous weld seam was formed with clusters of different phases. The area observed by both the XRD and SEM analyses shows a specific preferred crystallographic orientation towards the {111} family of planes. It is possible that such an occurrence in this region was caused by the high chemical activity of this family of planes. Previous research categorizes the chemical activity of the different crystallographic planes with the {111} family of planes being the most chemically active due to its low free surface energy, and the {100} family as the least chemically active due to its highest free surface energy [
21]. Another study confirms this and also suggests that the {111} family of planes exhibits the highest wettability potential [
22], which improves the chemical bonding, in this case, between the intermetallic compounds and the αAl matrix. Thermodynamically, this family of planes should suffice for the formation of the highest amount of chemically and energetically stable bonds with a low amount of internal stresses. Of course, the latter were not studied in the present research, and this is just a hypothesis.
Electron beam processes, particularly welding processes, are characterized by a high heat input derived from the high density of the accelerated beam of electrons [
23]. Due to the rapid and highly focused melting of the materials, high thermal gradients are observed [
24]. The theory of solidification processes suggests that thermal gradients (i.e., cooling rates) are of exceptionally high importance to the resultant structure and mechanical properties of the formed non-separable joint [
25]. The higher the cooling rate is, the lower the nucleation time is. This hinders the formation of secondary structures within the primary solid solution, such as eutectics and precipitates [
26]. In the present case, with the increase in the power of the electron beam, a noticeable increase in the input heat was observed, which resulted in slower cooling processes. This was confirmed by the SEM images, which show that, in the case of the lower power of 1800 W, poorly defined dendritic eutectic structures have formed. The increase in the input heat intensified and propagated the formation of intermetallic compounds of a similar, but yet much more pronounced structure. This is confirmed by the XRD experiments where the CuAl phase was detected in the case of the first two samples, and the EDS experiments which also detected the presence of the metastable Cu
9Al
4 phase. Such phases were absent during the investigations performed regarding the sample prepared using a beam power of 3000 W. Of course, it is crucial to mention that both the XRD and the EDS analyses were performed in highly localized areas, such that the absence or the presence of other intermetallic phases cannot be completely denied. The absence of intermetallic phases other than the CuAl
2 phase in the structure of the sample prepared with the highest power, however, definitively suggests that, even if other IMCs are present, their quantity is low enough to not be detected by either analysis.
The authors of [
27] have investigated the possibility of electron beam welding of copper and aluminum bimetallic plates with a similar power of the electron beam (3240 W). They report an accelerated formation of the CuAl
2 phase, which hinders the mechanical properties of the weld. They also suggest that the fracture mechanism is such that due to poor nucleation between the IMCs and the aluminum solid solution the fractures that form in the structure of the samples occur at the border between the IMCs and the crystals of the primary phase. Such results were also observed in the present work, where all fractures occurred between the fusion zone and the heat affected zone of either the aluminum or the copper plates. Li et al. [
28] have studied electron beam welded joints using a beam power of 1950 W. Unlike in the present work, they only observed the formation of the CuAl
2 phase and did not detect the presence of other IMCs. Their investigations, however, were performed on Cu and Al plates with a thickness of 3 mm, so full penetration of the electron beam was achieved in all cases. Furthermore, they report similar tensile properties, highly comparable to the ones obtained in this work. Otten et al. [
29] have also investigated the possibility of electron beam welding of 3 mm thick copper and aluminum plates with similar technological conditions as the ones investigated in [
28]. They, however, study the possibility of reducing the formation of IMCs by applying varying offsets of the electron beam towards the Al plate and achieve great success. However, the parasitic metastable Cu
9Al
4 phase was also present within the structure of the joints. A highly known problem of electron beam welding is the formation of pores either due to low miscibility or due to the breaking apart of Cu
xO
x or Al
xO
x phases, thus causing the release of oxygen. This effect was studied in detail by the authors of [
30], who report that different copper wires can have different oxygen absorption affinity, leading to the formation of pores within the weld seam. In the present work, no presence of gas cavities was detected while examining the samples using SEM.
The presence of intermetallic phases within the weld seam theoretically should increase its microhardness proportionally to their concentration. Previous investigations were performed [
31], and the hardness of different IMCs was determined. Evidently, the lowest possible microhardness belongs to the CuAl
2 phase, with a value of 324 HV. Considering the obtained microhardness of the weld seams during the present research, it is safe to assume that most of it is comprised specifically of the CuAl
2 phase, since both the average and the momentary microhardness values do not surpass 260 HV
0.05. One of the current hypotheses is that, in the case of the sample prepared with a power of 3000 W, the best miscibility was achieved, resulting in the successful formation of stable compounds in the form of the CuAl
2 phase and a minimal quantity of other IMCs. This was, as mentioned, supported by the XRD and EDS analyses, and it is also supported by the lowest microhardness of that sample, amongst others.
The effect of the formation of IMCs on the resultant microhardness and tensile strength is well visible. As mentioned above, the tensile properties of the first two samples prepared with a power of the electron beam of 1800 W and 2400 W was essentially nonexistent. Gathering accurate data on the exact tensile properties, particularly the ultimate tensile strength, the yield strength, and elongation was impossible due to the only partial penetration of the electron beam and the formation of small, shallow weld seams. Despite the poor miscibility between the aluminum matrix and the copper inclusions, in the case of using the lower values of the power of the electron beam, some intermetallic compounds were still formed. This increased the hardness of the weld seams, but did not result in the formation of cracks, neither along its length nor in the areas between the weld seams and the base materials. Regardless, the formation of IMCs is known to have a negative effect on the mechanical strength of weld seams [
32]. The bonding energy between the IMCs and the aluminum matrix is low, in comparison to the high bonding energy of the intermetallic compounds themselves [
33]. In addition, the presence of the latter, particularly inhomogeneously, along the cross-section of the weld seam also increases the internal stresses within the weld seam [
34]. On top of that, high stresses are normally generated in the border between the weld seam and the base materials due to the rapid change in the crystal structure. All of these factors have effects on the tensile properties of the formed welds, which, in the case of the samples prepared with a power of the electron beam of 1800 W and 2400 W, led to their immediate fracture, due to which, no measurable values were obtained. A much more stable (although still inhomogeneous) structure was observed in the case of the sample prepared with a beam power of 3000 W. Full penetration was observed, along with a preferred crystallographic orientation towards the {111} family of planes. The last is known to have the closest-packed structure with the lowest free surface energy, thus propagating the mobility of defects within the structure of the sample [
35]. This is a prerequisite for increased mobility of defects such as dislocations in the structure of the welded joint and, therefore, better plastic properties. As mentioned, in that case the highest chemical activity, the best bonding between the Al matrix and the IMCs is thus theoretically observed. As a result, the ductility of the sample prepared with a power of 3000 W is the highest. That, accompanied with the highest surface area of the weld seam, led to the formation of the strongest weld seam amongst the sample. The ultimate tensile strength of this sample is low, but it still might find some possible applications in the field of electrical engineering as far as the electrical properties (which were not studied in this research) are satisfactory. From a mechanical standpoint, the formed joint does not have satisfactory mechanical properties at all and is considered completely unsuccessful.
The present work studies the influence of the power of the electron beam during electron beam welding of dissimilar metals, in particular Cu and Al6082-T6 alloys. The structure of the weld seams was investigated, along with some mechanical properties. The results, as mentioned, were mostly unsatisfactory; however, a clear correlation between the formation of IMCs and their distribution along the cross-section of the formed weld seams was established. Evidently, the application of a higher-power electron beam is imminent. This has potential promise for the formation of high-strength Cu-Al joints. Since the relationship between the formation of IMCs and the power of the electron beam was established, and previous investigations established the optimal offset and oscillation radius, the only technological condition left for experimentation is the welding speed. Future studies need to be conducted in order to establish the best approach in that regard and its influence on the resultant microstructure and properties. Additionally, as hinted, Cu-Al joints can have great applications in the field of electrical engineering. For this, studies regarding the longevity of such joints, their electrical conductivity, and potential to corrosion resistance and galvanic corrosion should also be conducted. In terms of mechanical strength, it is highly possible that reducing the concentration of IMCs in the weld seam, and also improving their spread across its cross-section, would lead to an improvement in the mechanical properties. One such way this could be achieved is the application of a filler material between the copper and the aluminum plates. Some such studies have already been proposed [
13,
36].