1. Introduction
The environmental pollution and energy crisis are common challenges that human beings are facing. Moreover, the greenhouse effect resulting from the use of fossil fuels is an important problem in the development of society. Creating technology that converts carbon dioxide into valuable products is an attractive way to reduce the greenhouse effect [
1,
2]. More importantly, it is necessary to develop renewable energy to entirely solve the energy problem. Hydrogen energy is recognized as one of the ideal secondary energy sources due to its non-toxicity, non-polluting nature, high energy density, and renewability [
3]. However, efficient and safe hydrogen storage technology still sets a strict limit on the development of the utilization of hydrogen energy [
4]. Magnesium and its hydride are recognized as the optimal solid hydrogen storage material due to their high storage density of up to 7.6 wt%, good reversibility, and low cost [
5]. However, the high decomposition temperature and slow reaction kinetics of Mg/MgH
2 significantly limit its practical applications [
6].
To address these challenges, various methods have been explored to improve the hydrogen storage performance of magnesium-based materials, such as alloying with other elements [
7,
8,
9], doping catalysts [
10,
11,
12], nanosizing [
13,
14,
15], and so on. In materials science, it is known that symmetry plays a key role in hydrogen absorption and desorption in alloys, as it affects structural stability, atomic arrangements, and phase transitions. The alloying method consists of adding other elements to magnesium by alloy smelting to obtain alloys, thus enhancing the hydrogen storage performance of magnesium. Xie et al. [
16] prepared Mg-Ce alloys with various Ce contents, and the phase compositions, micro-structures, and hydrogen storage performance of the alloys were investigated. They found that the addition of Ce significantly improves the hydrogen absorption kinetic properties of the alloy, and the dehydrogenation temperature can be obviously reduced with the increase of Ce content. Yang et al. [
17] prepared several Mg
24Y
3M (M = Ni, Co, Cu, Al) ternary alloys, and the research results indicate that the alloy with different chemical components showed significantly different features in its microstructure and hydrogen storage properties. Mg
24Y
3Ni had the best hydrogen absorption and desorption kinetic performance. Doping catalysts is also an effective method to improve the hydrogen storage kinetic properties of Mg/MgH
2 [
18]. Currently, the catalysts used for hydrogen storage materials mainly include metals and alloys [
19,
20], oxides [
21,
22], halides [
23,
24], carbide [
11], and carbonaceous materials [
25]. The addition of transition metal elements or their inorganic compounds has proven to be an effective method for enhancing the hydrogen storage performance of magnesium-based materials. Oelerich et al. [
26] doped different transition metallic oxides (Sc
2O
3, TiO
2, V
2O
5, Cr
2O
3, Mn
2O
3, Fe
3O
4, CuO/Al
2O
3, and SiO
2) into MgH
2 by a simple ball-milling method, and the hydrogen storage measurement results indicate that all these oxide catalysts can improve the hydrogen absorption and desorption performance of MgH
2. Li et al. [
27] synthesized a nanocrystalline Ni@C composite using a self-templating method and then doped it into MgH
2 to investigate its catalytic impact on the hydrogen storage properties of MgH
2. The research results demonstrate that the dehydrogenation temperature of the composite was lowered by more than 74 °C compared to pure MgH
2.
In recent years, metal sulfides have aroused more attention due to their outstanding catalytic properties the in water–electrolytic hydrogen evolution and photocatalytic fields [
28,
29]. Moreover, metal sulfides also have unique catalytic effects in improving the hydrogen storage performance of magnesium-based materials. Yuan et al. [
30,
31,
32] compared the catalytic effects of CoS
2 and MoS
2 nano-particles on the hydrogen storage properties of Sm–Mg binary alloy. They found that both of the sulfides could obviously improve the hydrogenation and dehydrogenation kinetics of Sm–Mg alloy, and the composite catalyzed by MoS
2 exhibits better hydrogen storage properties. Wang et al. [
33] also synthesized NiS
2 particles by a one-step hydrothermal method, and various MgH
2–NiS
2 composites with different NiS
2 contents were prepared by mechanical ball-milling. The study indicates that the NiS
2 decomposed and transformed into MgS and Mg
2NiH
4 phases during the hydrogenation process, and the catalytic phases formed in situ significantly improved the hydrogenation and dehydrogenation kinetic properties of MgH
2. Based on previous studies, a Mg
95Ce
5 alloy was prepared by using the vacuum induction melting method in this study. Then TiS
2 was selected as a catalyst to dope into Mg
95Ce
5 alloy by using the mechanical ball-milling method to prepare a series of Mg
95Ce
5–
x wt% TiS
2 (
x = 0, 3, 5 and 10) composites with different TiS
2 contents. The effects of TiS
2 content on microstructure and hydrogen storage properties of Mg
95Ce
5–TiS
2 composites were studied, and the catalytic mechanism of the TiS
2 on hydrogen absorption and desorption reactions of Mg
95Ce
5 alloy was investigated in detail.
3. Results and Discussion
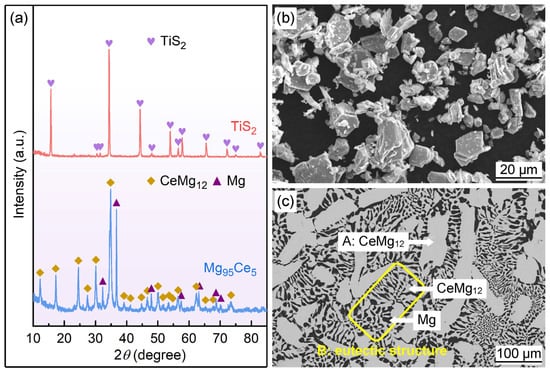
The phase compositions and microtopography features of the TiS
2 catalyst and Mg
95Ce
5 alloy were characterized using XRD and SEM analysis, and the results are illustrated in
Figure 1. It can be observed in
Figure 1a that the TiS
2 sample exhibits distinct diffraction peaks, indicating it is a typical crystalline structure.
Figure 1b presents the SEM image of TiS
2 powder. It can be seen that the TiS
2 particles exhibit a polygonal flake-like structure and particle size of 5~20 μm. The XRD analysis results in
Figure 1a indicate that the Mg
95Ce
5 alloy is composed of CeMg
12 and Mg phases, and the sharp diffraction peaks imply the alloy has good crystallinity.
Figure 1c shows the SEM image (backscattered electron image) of the Mg
95Ce
5 alloy ingot. It is evident that the alloy mainly consists of two structures: the one is a coarse and gray-white blocky phase (marked A), and the other is a eutectic structure composed of a striped or globular gray-white phase and a black phase (marked B). In general, elements with bigger atomic numbers appear brighter in backscattered electron images. Combined with XRD analysis and a Mg–Ce binary phase diagram [
34], we can conclude that the gray-white phase in areas A and B is CeMg
12, and the black phase in area B is Mg.
Mg
95Ce
5–TiS
2 composites with different TiS
2 content were prepared by the mechanical ball-milling method, and the XRD patterns of the composites are shown in
Figure 2. It can be observed that the ball-milling process has almost no influence on the phase compositions of the alloy, and the ball-milled Mg
95Ce
5 alloy still consists of CeMg
12 and Mg phases. However, the diffraction peaks of the ball-milled Mg
95Ce
5 alloy are broadened and weakened, which can be attribute to the lattice distortion and grain refinement resulted from the ball-milling process [
35]. Moreover,
Figure 2 also illustrates that the TiS
2 content has little effect on the phase composition of Mg
95Ce
5–TiS
2 composites. Meanwhile, the diffraction peaks of TiS
2 cannot be observed in the Mg
95Ce
5–TiS
2 composites, even when the TiS
2 content reaches 10 wt%. This may be due to the fact that the crystal structure of TiS
2 is destroyed under the high-impact energy in the ball-milling process [
36,
37].
Figure 3 shows the SEM images of the ball-milled Mg
95Ce
5–TiS
2 composites with different TiS
2 contents. The figure illustrates that these ball-milled composites contain irregular particles with a particle size of 20~50 μm. Increasing TiS
2 content appears slightly reduce the particle size of the alloy powders. This phenomenon may be ascribed to the added grinding effect of the TiS
2.
To explore the phase transformations of the Mg
95Ce
5–TiS
2 composites during hydrogenation processes, XRD tests were conducted on the hydrogenated and dehydrogenated composite materials. It can be seen from
Figure 4a that the hydrogenated Mg
95Ce
5 sample mainly contained MgH
2 and CeH
2.73. Clearly, the CeMg
12 and Mg phases in the alloy were completely transformed into MgH
2 and CeH
2.73 in the hydrogen absorption process. This result is consistent with the hydrogenation process of Mg
3RE (RE represents rare earths) intermetallic compounds [
38]. Moreover, the diffraction peaks of CeS
2 and TiH
1.5 could be detected in the hydrogenated Mg
95Ce
5–TiS
2 samples. Therefore, it can be asserted that the TiS
2 decomposed and transformed in situ into CeS
2 and TiH
1.5 during hydrogenation. The appearance of CeS
2 suggests that sulfur is more likely to combine with cerium rather than magnesium to form a CeS
2 compound.
Figure 4b shows the XRD patterns of the dehydrogenated Mg
95Ce
5–TiS
2 composites. The figure shows that the diffraction peaks of MgH
2 disappeared to be replaced by the diffraction peaks of Mg. Obviously, the MgH
2 in hydrogenated samples can be completely decomposed and release hydrogen during dehydrogenation process. Moreover, there is no chemical reaction occurs in the CeH
2.73, CeS
2 and TiH
1.5 phases during hydrogen absorption and desorption cycles. The CeH
2.73 and TiH
1.5 has good chemical stability and there is decomposition reaction only at higher temperatures [
39,
40]. Despite of this, the in-situ formed CeH
2.73, CeS
2 and TiH
1.5 can dramatically catalyze the hydrogen absorption and desorption reactions.
After five hydrogen absorption and desorption cycles, the Mg
95Ce
5–TiS
2 composites could achieve a stable reaction kinetics. Then the TPD curves, isothermal hydrogen absorption/desorption kinetic curves, and
p–
c–
T curves could be measured.
Figure 5 exhibits the TPD curves of the composite samples at a heating rate of 3 °C min
−1. The dehydrogenation peak temperature of the Mg
95Ce
5 alloy was approximately 389.5 °C, and the entire desorption reaction was completed when the temperature exceeded 450 °C. Notably, the dehydrogenation temperature of the Mg
95Ce
5–TiS
2 composite material was much lower than that of the Mg
95Ce
5 alloy sample, and the dehydrogenation peak temperature of the materials gradually decreased to 329.7 °C when the TiS
2 content increased to 10 wt%. However, the addition of TiS
2 increased the total weight of the Mg
95Ce
5–TiS
2 composites and inevitably reduced its effective hydrogen storage capacity.
Figure 6 presents the hydrogen absorption and desorption curves of the Mg
95Ce
5–TiS
2 composites at various temperatures. From the hydrogen absorption kinetic curves shown in
Figure 6a–c, we can observe that all the samples exhibited good hydrogen absorption kinetics at 380 °C. That is, the TiS
2 catalyst did not have a significant impact on the hydrogen absorption kinetics of the composite at higher temperatures. With the decreases of operating temperature, the hydrogen absorption rate of the pure alloy material began to be limited. For example, the Mg
95Ce
5–10 TiS
2 composite reached full hydrogenation saturation within 75 min at 250 °C, whereas the Mg
95Ce
5 only reached full saturation at around 300 min. From the hydrogen desorption kinetic curves shown in
Figure 6d–f, it can be seen that the hydrogen desorption kinetics of the Mg
95Ce
5–TiS
2 composites were also much faster than those of the Mg
95Ce
5 alloy. More importantly, increasing TiS
2 content significantly increased the hydrogen desorption rate. However, the maximal hydrogen absorption and desorption capacities of the composites gradually decreased with the increase of TiS
2 content, which coincided with the results of the TPD test.
Generally, the dehydrogenation reaction of magnesium hydride follows the nucleation growth model [
41]. The Johnson–Mehl–Avrami–Kolmogorov (JMAK) formula can be used to fit the dehydrogenation kinetic curves of the Mg
95Ce
5–TiS
2 composites for a better understanding of the dehydrogenation mechanism. The JMAK formula can be expressed as [
42]:
where α represents the extent of the dehydrogenation reaction at moment t, n denotes the Avrami exponent, and k is the reaction rate constant, which depends on temperature. The kinetic curve of hydrogen desorption at various temperatures was fitted with a linear JMAK equation by plotting ln[−ln(1 − α)] against lnt. In this plot, n corresponds to the fitting slope, while nlnk is the intercept. The JMAK fitting results are illustrated in Figure 7a–d, where a clear linear relationship is observed in each plot, indicating that the dehydrogenation reaction of the Mg95Ce5–TiS2 composite material conforms to the JMAK model. The calculated values of n and lnk are presented in Table 1. The values of n for the ball-milled Mg95Ce5 alloy range from 3.305 to 3.696, suggesting that three-dimensional interfacial growth determines the dehydrogenation process [19]. After doping with TiS2, the Avrami coefficients of the samples varied from 1.341 to 1.788, implying that the dehydrogenation process of the TiS2-catalyzed Mg95Ce5–TiS2 composites could be described using a zero-nucleation rate mechanism and the reaction was limited by the one-dimensional diffusion of the elements [27]. It is obvious that the CeH2.73, CeS2, and TiH1.5 formed in situ play the role of nucleating agents during the dehydrogenation process of the Mg95Ce5–TiS2 composites and significantly accelerate the hydrogen desorption kinetic performance of MgH2 in the composites.
The dehydrogenation kinetic performance is typically characterized by the apparent dehydrogenation activation energy (
Ea), which can be calculated using the Arrhenius equation [
43]:
where lnk can be determined from Equation (1), Ea represents the apparent dehydrogenation activation energy (kJ mol−1), R denotes the molar gas constant (8.314 J mol−1 K−1), T is the thermodynamic temperature in Kelvin, and k0 is a constant. Figure 7e illustrates the Arrhenius fitting curves of lnk plotted against 1000/T. The Ea values can be derived from the slope of the fitted lines, and these obtained values are also compared in Figure 7f. We notice that the Ea value for dehydrogenation reactions decreased from 117.4 kJ mol−1 to 75.0 kJ mol−1 with the increase of TiS2 content from 0 to 10 wt%. The Ea value of the Mg95Ce5–TiS2 sample in this study wass much lower than that of the MgH2/TiO2 composites (106.7 kJ mol−1) [44] and MgH2–Ni/TCN material (82.6 kJ mol−1) [45] reported before. Therefore, we can conclude that adding TiS2 to Mg95Ce5 alloy can obviously decrease the dehydrogenation activation energy and therefore significantly improves the hydrogen desorption kinetic performance of the alloy.
To further investigate the impact of TiS
2 on the hydrogen storage thermodynamics of the Mg
95Ce
5 alloy, the
p–
c–
T curves of the samples at different temperatures were measured, and the results are illustrated in
Figure 8a–d. It is evident that all the
p–
c–
T curves of the samples exhibited only one flat desorption plateau at each temperature, indicating that only one phase, namely Mg/MgH
2, is involved in the reversible hydrogenation cycling. The changes in enthalpy (Δ
H) and entropy (Δ
S) related to the hydrogen absorption and desorption reactions of the composites can be calculated using the van’t Hoff equation [
46]:
Here
peq is the dehydrogenation equilibrium pressure (MPa),
p0 is the standard atmospheric pressure (0.101325 MPa),
R represents the gas constant (8.314 J mol
−1 K
−1), and
T represents the experimental temperature (K). The van’t Hoff plots for the hydrogenation and dehydrogenation reactions of the Mg
95Ce
5–TiS
2 composites are shown in
Figure 8e,f, and the calculated Δ
H and Δ
S values are listed in
Table 2. Notably, there is no significant difference in Δ
H and Δ
S values, indicating that the TiS
2 had little effect on the hydrogen absorption and desorption thermodynamic properties of Mg
95Ce
5 alloy. This is mainly because the de/re-hydrogenation reactions in the Mg
95Ce
5–TiS
2 composites were Mg + H
2 ↔ MgH
2, and the catalyst could not markedly affect the hydrogen absorption and desorption reaction process.