1. Introduction
Capsicum species (Solanaceae) are important economic crops widely cultivated worldwide, particularly in Pakistan, India, China, Thailand, and Mexico [
1,
2,
3]. The main cultivated types are
Capsicum annuum L. and
Capsicum chinense L., with
C. annuum being the primary cultivar in China [
4,
5]. The southwest region of China, encompassing Sichuan, Chongqing, Guizhou, and Yunnan, serves as a major hub for cultivating and consuming peppers [
6]. Pepper planting in Guizhou spanned 0.36 million hm
2 in 2021, ranking first in China [
7]. However, due to the development and smelting of mineral resources, and the weathering and leaching of carbonate rock areas, heavy metal pollution in farmland has become a serious issue in Guizhou [
8,
9,
10]. Cadmium (Cd) is one of agricultural soils’ most serious heavy metal pollutants. Its transfer from soil to plants is the primary route of human exposure, and it poses significant health risks [
11]. This contamination presents a significant challenge for developing pollution-free agriculture and sustainable land use in Guizhou [
12]. Therefore, developing Cd-resistant pepper cultivars with low heavy metal content is crucial for ensuring food safety and protecting the soil ecosystem [
13,
14,
15].
Pest damage is a key issue affecting the sustainable development of pepper agriculture. Herbivorous insects, which feed on living plant tissues, play a key role in the transmission and accumulation of Cd. Numerous studies have extensively investigated the movement and toxic effects of Cd from soil to plants and herbivorous insects [
16,
17,
18]. As an abiotic environmental factor, Cd not only exerts toxic effects on insect development but also influences the effectiveness of integrated pest management methods, such as biological control (utilizing insect pathogens, parasitoids, and predators) and chemical control (applying chemical insecticides and plant-based toxins) [
19]. After being subjected to heavy metal stress, plants typically exhibit increased toxicity to herbivorous pests [
20,
21,
22]. This phenomenon has been observed in
Plutella xylostella consuming Ni-contaminated
Streptanthus polygaloides [
23],
Pieris napi consuming Cd- and Zn-contaminated
Arabidopsis halleri [
24], and
Spodoptera litura consuming Cd-contaminated
Brassica campestris [
25]. This effect is typically due to the production of secondary metabolites as organic defenses alongside direct toxicity from heavy metals, which can create dual toxicity. However, the combined toxic effects of plant toxins and heavy metals may accelerate the development of pests’ adaptability [
26].
The gut microbiota is essential for the ecological adaptation of herbivorous pests, significantly impacting insect development, reproduction, behavior, survival, and stress resistance (including tolerance to insecticides, plant toxins, and heavy metals) [
8,
27,
28,
29]. Current research indicates that exposure to heavy metals disrupts the structure of insect gut microbiota, thereby affecting their metabolic and physiological functions within the host [
30,
31,
32]. For instance, after feeding on artificial diets contaminated with varying concentrations of Cd, the abundance of beneficial gut microbiota (
Serratia,
Weissella, and
Aeroonas) significantly decreased in
Lymantria dispar larvae, while the abundance of pathogenic gut microbiota (
Cutibacterium,
Stenotrophomonas, and
Gardnerella) significantly increased [
33]. Additionally, certain bacterial species in the insect gut microbiota can enhance the host’s tolerance to exogenous toxic substances, although the primary mechanism does not involve direct detoxification by the gut microbiota.
Enterococcus identified from the
Plutella xylostella’ gut has been shown to boost the moth’s immune system, thereby increasing its resistance to insecticides [
34]. Zhang et al. [
35] also demonstrated that the intestinal bacteria of
Curculio chinensis interacts with the chemical compounds found in camellia seeds, with the gut microbiota mediating the degradation of saponins.
Spodoptera litura (Lepidopteran: Noctuidae) is one of the main pests affecting pepper seedlings. In recent years, the damage to pepper caused by
S. litura has risen due to the expansion of pepper cultivation and favorable climatic conditions [
36,
37]. However, the extensive, frequent, and unreasonable use of chemical pesticides has resulted in varying resistance levels to multiple pesticide types in
S. litura [
11,
38]. In our previous study, compared with artificial diets, plants grown in Cd-contaminated soil exerted more substantial toxic effects on
S. litura larvae, leading to physiological and biochemical changes related to the insect’s stress resistance. This change may accelerate the development of insect adaptability in heavy metal-contaminated farmland [
25,
26,
39].
In this study, S. litura larvae were subjected to two Cd-contaminated chili pepper cultivars Capsicum annuum; we determined the impacts of Cd stress via Cd-contaminated peppers on their growth indices and nutritional indices. Afterwards, we analyzed the influence of Cd on the microbiota in S. litura. The objectives of this study were to (1) investigate the effects of Cd-contaminated pepper cultivars on the growth indices and nutritional indices of S. litura larvae and (2) elucidate the toxic effects of Cd-contaminated pepper cultivars on the gut microbiota of S. litura. This study will offer new insights into gut microbiota response in herbivorous pests to plant-derived heavy metal contamination.
2. Materials and Methods
2.1. Plant
The seeds of two pepper
Capsicum annuum L. hybrids, pod pepper Chiyan (CY, high-Cd cultivar) and line pepper Tianlanse (TLS, low-Cd cultivar), were obtained from the Shandong Shouhe Seed Co., Ltd. (Weifang, China). The Cd contents in pepper leaves are shown in
Supplementary Data Figure S1. The seeds were sterilized on the surface by soaking in 5% NaClO
3 for 5 min, then rinsed thoroughly with distilled water. Afterward, they were placed on moist filter paper and germinated in complete darkness at 25 °C for 48 h. Following germination, uniform seedlings were selected and transplanted into seedling trays containing a non-Cd substrate. The seedlings were cultivated in an artificial climate chamber set to 25 ± 5 °C, with a 16 h light/8 h dark photoperiod, and maintained soil moisture at 70%. Once the seedlings reached the four-leaf stage, they were transferred into plastic pots containing soil with varying concentrations of Cd, with 30 replicates for each Cd treatment. After 60 days of cultivation, the leaves were harvested to feed
S. litura larvae. We also measured the Cd concentration in pepper leaves using the same method as described in [
39].
2.2. Insect
S. litura was obtained from a vegetable field in Huaxi District, Guiyang, Guizhou Province, and subsequently reared indoors. Prior to the experiment, the larvae were not subjected to any toxic chemicals (such as acaricides, fungicides, and antibiotics). The newly hatched larvae were reared on an artificial diet as described by [
40]. The rearing process was conducted in a climatic chamber under controlled conditions, maintained at a 14 h light/10 h dark photoperiod and 26 °C with 60% relative humidity.
2.3. Experimental Design
The potted soil was taken from the top layer (5–25 cm) of the pepper field in Huaxi District, Guiyang, China. After collection, the soil was treated indoors as described in [
25]. The pot experiment was performed in the greenhouse of Guizhou Normal University, China. The planting period was from March to July 2024. A total of 400 g of soil was thoroughly mixed with 5 g of compound fertilizer and transferred into plastic pots (11.8 cm in height and 12.3 cm in diameter). A prepared CdCl
2 solution (Macklin, Shanghai, China) was then added to the soil to achieve the concentrations of 0, 2.5, 5, and 10 mg/kg Cd. The soil was thoroughly mixed and watered with distilled water to maintain 70% soil moisture. Pepper seedlings were relocated into plastic pots after a 7 d stabilization period.
2.4. Impacts of Cd-Contaminated Pepper on the Survival Rate of S. litura
Around 200 eggs were placed in a 15 cm sterile plastic box. After hatching, fresh artificial diets were provided daily. When the larvae reached the 2nd instar, 30 larvae of similar size (0.035–0.045 g) from each treatment were moved to 4 cm sterile plastic cups. Each larva was provided with an ample supply of pepper leaves from the various Cd treatments, with fresh leaves supplied daily. The number of surviving larvae was recorded daily. Three replicates were conducted for each Cd treatment, with 30 insects in each replicate.
2.5. Impacts of Cd-Contaminated Pepper on Larval Weight and Nutritional Indices of S. litura
Larvae reared individually are described in
Section 2.4. The nutritional processes in Lepidoptera larvae were analyzed using the nutritional indices established by [
40]. After recording the larvae’s initial weight and introducing fresh Cd-treated pepper leaves, 15 2nd instar larvae from each treatment were individually placed into 4 cm sterile plastic cups. Each bioassay was conducted in triplicate. The weights of the larvae, feces, and remaining leaves after feeding were recorded for 10 d. Nutritional indices, including relative consumption rate (RCR) (g/g/d), approximate digestibility (AD) (%), efficiency of conversion of ingested food (ECI), efficiency of conversion of digested food (ECD) (%) and relative growth rate (RGR) (g/g/d), were assessed and determined following the method described in [
41].
2.6. DNA Extraction, Sequencing, and Bioinformatics Analysis
Larvae fed pepper leaves treated with Cd for 10 days were collected. After a 10 min ice bath, the larvae were treated with 75% ethanol for 120 s on the surface and then fixed on a sterile wax plate. The bodies of larvae were longitudinally dissected using sterile surgical scissors, rinsed in PBS buffer, and the entire gut tissues were collected in a 1.5 mL sterile Eppendorf tube and frozen in liquid nitrogen immediately. Five guts were combined to form one biological sample in each treatment group, and this process was repeated three times.
DNA extraction of the intestinal bacteria used an E.Z.N.A.® Soil DNA Kit (Omega, Norcross, GA, USA) following the instructions of the manufacturer. DNA yield and quality were evaluated using Qubit 4.0 (Thermo Fisher Scientific, Waltham, MA, USA). 16S rRNA gene amplicon sequencing (V5–V7) was performed by Shanghai Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China) with specific bacterial primers barcode 799F (5′-AACMGGATTAGATACC0G-3′) and 1193R (5′-ACGTCATCCCCACCTTCC-3′). Amplicons were sequenced on the Illumina Nextseq2000 platform (San Diego, CA, USA).
The raw data from both ends were imported into QIIME2 (v.2022.2.0), where the bacterial type was defined as a single-end sequence after sequencing. 16S rRNA gene amplicon data were processed using the “DADA2” pipeline in R (v.3.3.1) and the Silva database (v.138) (
https://www.arb-silva.de/, (accessed on 8 July 2024)) [
42]. We distinguished the data of each sample based on the barcode sequences, performed length filtering and orientation correction, and retained bacterial sequences between 1000 and 1800 bp. We performed quality control and generated amplicon sequence variant (ASV) signature tables that were calculated according to [
43]. The optimized HiFi reads were grouped into operational taxonomic units (OTUs) using UPARSE (v.7.1) at a 97% sequence similarity level [
44,
45]. A total of 1,513,290 reads were obtained from sequencing with an average read length of 376 bp. The acquired sequences were matched to 146 OTUs, with 111 OTUs identified in the CY samples and 94 OTUs in the TLS samples. These OTUs belonged to 14 phyla, 104 genera, and 120 species (
Supplementary Data Tables S1 and S2). The raw sequencing reads for intestinal bacteria are available in NCBI Biological Programs (PRJNA1177675).
Alpha diversity (including the sum of the observed number of species (Sobs), Shannon–Weaver diversity index (Shannon), Simpson’s index of diversity (Simpson), abundance-based coverage estimator (ACE), and abundance-based estimator of expected species richness (Chao1) of the intestinal bacteria community were determined by Mothur (v.1.30.2) [
46]. The beta diversity analysis was analyzed by principal co-ordinates (PCoA) analysis using the Vegan (v.2.5-3) package in R. The Kruskal–Wallis H test from the stats package in R (v.3.3.1) and the scipy package (v.1.0.0) in Python were used to perform hypothesis testing on species across different microbial communities based on the obtained community abundance data, evaluate the significance level of species abundance differences, and identify significant intergroup differences at the genus level. The PICRUSt (v.1.1.0) database (
http://picrust.github.io/picrust/ (accessed on 8 July 2024)) was employed to predict the functional capacity of the bacterial community [
47].
2.7. Statistical Analysis
Statistical analysis was carried out using IBM SPSS statistical software (v.21.0, SPSS, Inc., Chicago, IL, USA). Descriptive statistics were applied to test the data for normality, followed by exploration. One-way analyses of variance (ANOVAs) followed by Tukey’s HSD test and generalized linear models (GzLMs) were used to determine significant differences in the growth indices (the survival rate and the body weight of the 10 d larvae) and nutritional indices (RCR, RGR, ECI, ECD, and AD). The Pearson correlation coefficient was employed to analyze the correlations between the larvae’s gut microbiota and Cd accumulation in pepper leaves, as well as their biological parameters and nutritional indices. Before analysis, percentages of survival and nutritional indices were transformed using the arcsine square root method.
4. Discussion
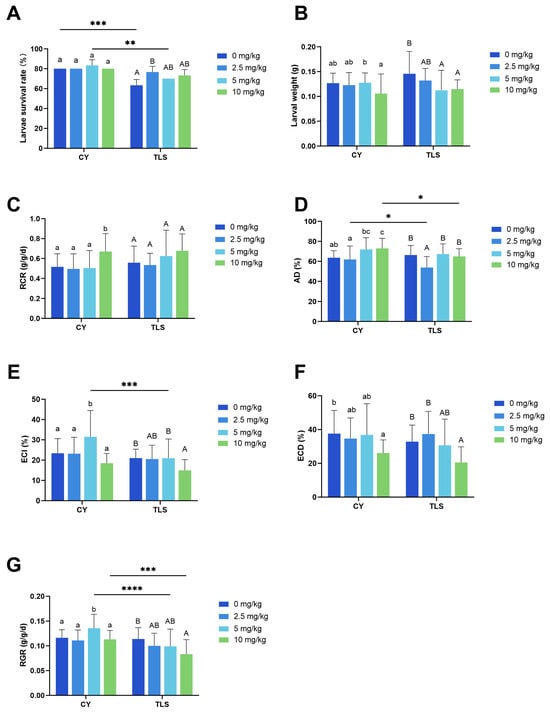
Overall, our study revealed differences in the growth, food utilization, and gut microbiota of
S. litura larvae between two pepper cultivars exposed to different concentrations of Cd pollution. We observed that the toxicity of CY is lower than that of TLS, primarily reflected in differences in larvae survival rates. GLM analysis also correlated with pepper cultivars and larval survival (
Figure 1A and
Table 2). The reason for this phenomenon may lie in the differences in plant toxins between the two pepper cultivars, as noted in previous studies with cayenne pepper (FXBX) and the Chao tian chili pepper (BLTY2) on
S. litura larvae [
36]. However, after Cd contamination, the degree of toxicity to larvae exhibited by the two pepper cultivars differed. In CY, 10 mg/kg Cd-treated pepper decreased the growth and food utilization (higher RCR and AD and lower ECD) of larvae, whereas 5 mg/kg Cd treatment increased the ECI and RGR. In TLS, we did not observe any promotive effects. The toxicity of Cd to larvae increased with the concentration (
Figure 1 and
Table 1). We hypothesize that one of the reasons for the toxicity difference may be due to the varying changes in the defense capabilities of different pepper cultivars after Cd poisoning. Reports indicate that the volatiles released and secondary metabolites produced can differ among cultivars of the same species due to individual variations, with these differences potentially linked to genetic factors and various biotic and abiotic elements, such as the plant’s nutritional status, stress conditions (e.g., heavy metals), and phenological stages [
48]. Our previous research also indicated that Cd stress can improve the insect resistance of cabbages by increasing leaf total phenolics, flavonoids, and repellent volatile organic compounds (VOCs) and reducing soluble sugar and attractive VOCs [
26]. Further research is needed to identify the change in secondary metabolites associated with insect growth and food utilization under Cd pollution.
The gut microbiota of insects performs essential symbiotic functions, such as enhancing host nutrition, aiding in the digestion of food, degrading toxins ingested with the diet, and offering protection against pathogens [
6,
27,
49]. The gut microbiota of insects also faces selective pressures in natural conditions. Its composition may be influenced by dietary changes, food scarcity, and exposure to toxic substances (e.g., heavy metals, pesticides, and plant toxins) [
50,
51]. Insects absorb heavy metals from the environment through ingestion, primarily accumulating in their intestines, which is bound to impact the intestinal microbiota of insects. The present study showed no significant difference in Chao1 and ACE after consuming different pepper cultivars contaminated with Cd. However, there were differences between Shannon and Simpson indices in terms of cultivars. At the same Cd concentration, the Shannon index of CY was higher than that of TLS, while the Simpson index showed the opposite trend. GzLM results also revealed that pepper cultivars had an enormously significant effect on the Shannon and Simpson indices. These results further suggest that the differences between the pepper cultivars primarily cause changes in the diversity of gut microbial communities in the larvae.
A large number of studies have demonstrated that the gut microbiota of insects changes at the genus level when subjected to heavy metal stress [
29,
32]. In this study, we investigated the changes in gut microbiota composition in response to Cd-treated pepper leaves in
S. litura. At the genus level,
Enterococcus has the highest relative abundance in
S. litura larvae, similar to findings in
Chilo suppressalis [
52] and
Bombyx mori [
53].
Enterococcus was considered essential for metabolic adaptation to pathogenic or plant toxins and for anti-herbivore defense in
B. mori,
Helicoverpa zea, and
Porthetria dispar [
54,
55]. Vilanova et al. [
56] also showed that
Enterococcus dominates the gut microbiota of two specialized Lepidoptera species,
Hyles euphorbiae and
Brithys crini, which primarily feed on
Euphorbia sp., which is rich in latex, and
Pancratium maritimum, which are abundant in alkaloids. In our study, the higher relative abundance of
Enterococcus may be due to the need to detoxify the toxins (such as capsaicin) in peppers. The relative abundance of
Enterococcus in TLS was higher across all treatments than CY, and Pearson correlation analysis also demonstrated that
Enterococcus was significantly positively correlated with the RCR of larvae but negatively correlated with survival rate (
Figure 4), which may be attributed to the toxin levels in different pepper cultivars. We also found that the
Pluralibacter of larvae feeding on CY was higher at the same Cd concentration than that feeding on TLS. Pearson correlation analysis showed that
Pluralibacter had a significant negative correlation with the RCR and a significant positive correlation with survival rate (
Figure 4).
Pluralibacter has been found to contribute to biosynthetic pathways for B vitamins and code for a methionine-specific ABC transporter complex (MetNIQ) in
Haementeria sp. [
57]. At the same time, certain
Pluralibacter species are likely opportunistic microbes that exploit a compromised immune system or infections triggered by parasites in honey bees [
58]. Therefore, the functional identification of
Pluralibacter requires validation through future experiments.
5. Conclusions
Our study shows that chili peppers contaminated with varying concentrations of Cd influence the growth, development, nutritional indices, and gut microbiota of S. litura larvae, with effects differing by pepper cultivar. Larvae fed on Cd-contaminated CY experienced a lower degree of toxicity. Notably, under the 5 mg/kg Cd treatment in CY, the larvae exhibited a significantly higher survival rate, AD, ECI, ECD, and RGR than other treatments, indicating a promotive effect. At the same Cd concentration, the Shannon index was higher and the Simpson index was lower in CY than in TLS, suggesting that the microbiota diversity in larvae fed CY was higher than in that fed TLS. Firmicutes and Proteobacteria were the dominant phyla in the larvae gut microbiota, with Enterococcus and Pluralibacter being the dominant genera. Compared to CY, larvae feeding on Cd-contaminated TLS showed a decreased relative abundance of Pluralibacter and an increased abundance of Enterococcus. The primary functions of these microbial communities were related to metabolic pathways, followed by ‘biosynthesis of secondary metabolites’, ‘microbial metabolism in diverse environments’, ‘ABC transporters’, and ‘the phosphotransferase system’. These microbial shifts may partly explain the differences in toxicity observed in larvae feeding on different Cd-contaminated pepper cultivars.