1. Introduction
Protein is an essential ingredient of aquatic feeds given that it is a key source of metabolically active substances and amino acids needed for protein synthesis [
1,
2,
3]. An essential metric for assessing the cost-effectiveness and quality of feed formulations is protein content [
4]. Protein can be used as a partial source of energy when energy substances (fats and carbohydrates) are in short supply in the feed. It has been found that a lack of or insufficiency in protein in the feed can lead to stunted growth [
5,
6]. Yet, a great deal of protein in the diet can lead to excessive proteolytic metabolism for energy consumption, which can negatively impact fish health and the environment [
7,
8,
9] and lead to the decrease in the protein efficiency rate [
10]. For this reason, modern aquafeed companies typically lower the amount of protein in the diet to save costs and increase farming efficiency. Accordingly, the determination of the optimum protein requirement in feeds is of great importance for both fish nutritional studies and aquaculture interests.
It is widely recognized that dietary lipids, as macronutrients, are a main source of supplying the energy requirements of aquatic animals [
11]. According to a previous study, the availability of non-protein substances and the protein levels in the diet are related to the utilization of feed protein [
12]. This implies that lipids are an important nutrient for providing fish with the energy they need to survive and for maintaining the normal physiological functions of fatty acids [
13,
14]. The digestion and absorption of feed fats require blood circulation for transportation to other tissues and organs, and the stored fats in the fish body need to be mobilized by the transport action of the blood. Consequently, the level of blood fat in fish can reflect, to some extent, the status of fat metabolism in the whole fish body [
15]. Fish commonly suffer from metabolic disorders and reduced feed conversion rates caused by deficiencies or insufficiencies of fat in the feed [
16]. Nevertheless, excessive fat content in feed can lead to oxidative deterioration of the feed, excessive fat deposition in the fish, and reduced immune resistance to disease [
17]. This adversely affects fish health status and growth, making it imperative that the fat requirements in feeds receive attention.
The protein-sparing effect, which occurs when fat is used instead of protein as an energy source, can lower protein intake through catabolism [
3,
18]. In the case of insufficient fat within the supply, part of the protein in the feed is utilized to provide the energy needed for metabolism and tissue formation [
19]. Nevertheless, high levels of fat in the feed may hinder fish feeding and growth, as evidenced by fat accumulation, growth decline, and reduced digestion and absorption capacity [
20,
21,
22]. In general, an optimized protein and fat content in feeds can boost growth, lower nitrogen content, and simultaneously diminish feed costs [
2,
18,
23]. Previous studies have identified some ideal protein and fat levels for aquatic animals, such as
Channa maculate [
18,
24],
Misgurnus anguillicaudatus [
25],
Colossoma macropomum [
3],
Dentex dentex [
2], and
Macrobrachium americanum [
19], encouraging the critical role of comprehensive investigations into dietary protein and lipid inclusion levels. Therefore, the protein and fat levels in feeds are the most essential parameters in feed formulation development.
Furong crucian carp is a finfish species chosen and propagated by the Hunan Fisheries Science Institute. Furong crucian carp features the characteristics of fast growth, excellent meat tenderness, and high resistance to adversity [
26]. Although previous studies have determined the appropriate levels of protein and fat for carp feed [
16,
27,
28], excessive protein and lipid inclusion levels in aquafeed may contribute to high metabolic stress in fish, which in turn hinders rapid growth regeneration, nutrient uptake, and metabolic functions [
7,
29]. As is often the case, the optimal level of dietary protein and lipids depends on various variables such as aquatic species, feeding rate, protein source, age, maturity stage, and habitat. Furong crucian carp is a hybrid species, and its actual requirements for feed nutrients may be different from those of other carp species. Currently, research on major nutrients in Furong crucian carp feeds remains unknown, encouraging further study on the appropriate fat and protein levels and their interactions. Hence, this study was designed to assess the impacts of different fat and protein inclusion levels in Furong crucian carp feed on growth performance, whole-fish proximate composition, digestive capacities, and blood biochemical indices, which may be used to guide the selection of culture techniques in production.
2. Materials and Methods
2.1. Ethic Statement
Hunan Fisheries Science Institute approved all experiment procedures, which adhered to the ethical standards for the care and use of laboratory animals (grant number: HNFI20210322).
2.2. Experimental Diet and Feeding Management
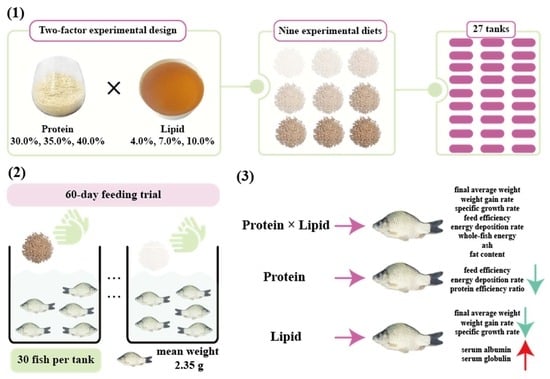
A two-factor design of dietary protein and fat levels was used in this experiment, with three protein (30.0, 35.0, and 40.0%) and three lipid (4.0, 7.0, and 10.0%) levels each configured into nine experimental feeds (
Table 1). The selection of dietary protein and fat level used in this study referred to the previous studies on carp [
30,
31,
32,
33]. The experimental feed was a granular feed prepared at the Hunan Fisheries Science Institute (Changsha City, Hunan Province, China), of which the feed ingredients referenced those of the crucian carp feed [
32]. Fish meal, rapeseed meal, and soybean meal were the main protein sources in the experimental diet. Soybean oil was the predominant source of dietary fat. The sugar source and adhesive were wheat middling and corn starch, respectively. The procedure for preparing feed is mentioned in the earlier literature [
34]. Specifically, the ingredients were mixed using the gradual expansion method following being ground and then sieved through a 60-mesh sieve. Upon blending, the feed passed through a feed pelletizer to yield pellets with a particle size of around 1.2 mm. The pellets were then allowed to air dry in a cool environment before being refrigerated at −20 °C. The proximate nutritional composition of experimental diets was measured using the previously described methodology [
35], the crude protein and crude fat contents of the experimental feeds were tested with reference to the Soxhlet extraction method (GB/T 6433-2006 [
36]) and the Kjeldahl method (GB/T 6432-1994 [
37]), respectively, which follow Chinese national standards.
The experiment was carried out at the Hunan Fisheries Science Institute. The whole feeding trial was undertaken in an indoor glass tank (100 × 50 × 50 cm) recirculating water aquaculture system. No feed was given during the first 2 days of releasing the fish into tanks and the whole domestication lasted for 7 days. In total, 540 carp, with an initial body weight of 2.36 (±0.02) g by group weighing, were assigned into 9 groups and fed with experimental diets for 60 days. The whole feeding trial was carried out in 27 glass tanks, with each group consisting of 3 replicates (tanks) and housing 20 fish per tank. The pH (7.6 ± 0.6), temperature (28.4 ± 3.5 °C) (measured using a thermometer), ammonia (≤0.2 mg·L−1) (measured with a portable colorimeter, LH-M900, CHINCAN, Zhejiang, China), and dissolved oxygen (7.2 ± 0.3 mg·L−1) in the culture water were all recorded. Throughout the feeding trial, the quality of the water did not change.
2.3. Sample Collection
At the final sampling, the carp were anesthetized with eugenol (C
10H
12O
2, 1:12,000) according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals at the end of the 60-day feeding trial. Specifically, eugenol and ethanol were mixed at a ratio of 1:8 to formulate an anesthetic agent for use. The mixture was splashed into the bucket in which the fish were placed, ensuring that the concentration of eugenol was around 50 mg/L. The anesthesia lasted for about 1 min, after which the anaesthetized fish were dissected. Following the selection of six carp at random from each tank, samples of the midgut, liver, and viscera were taken. For the nutritional study of the entire body, another three carp per tank were further gathered using the previously described methodology [
38]. A sterile syringe of 1 mL was utilized to extract blood from the tail vein of six carp in each group, with four 2 mL EP tubes collected from each group and stored at 4 °C overnight. The blood was then centrifuged for 10 min at 4000 r/min, and the supernatant was collected and kept at −80 °C.
The fish number, feed intake, and final weight (FW, g) of fish per tank were counted and recorded by group at the end of the feeding trial, and survival rate (SR, %), specific growth rate (SGR, %/d), weight gain rate (WGR, %), and feed efficiency (FE, %) were calculated according to the formulas of the previous report [
38]. Furthermore, the protein efficiency rate (PER, %) and energy deposition rate (EDR, %) were figured out as described above [
39,
40]. Specifically, the formulas used to calculate growth-related parameters are listed below:
In the above equations, W1 is the total weight of fish at the beginning of the experiment (g); W2 is the total weight of fish at the end of the experiment (g); N1 is the initial number of fish; N2 is the final number of fish; W3 is the liver weight of the selected individual fish (g); W4 is the viscera weight of the selected individual fish (g); Wt is the weight of the selected individual fish (g); Wf is the weight of the feed intake (g); Wp is the crude protein content of the feed (g); E2 is the energy of the selected individual fish (MJ/kg, air-dried basis); E1 is the energy of the feed ingested by the selected individual fish (MJ/kg, air-dried basis); L is the body length (cm).
2.4. Metabolites and Enzymes Assays
Intestinal and hepatic enzyme activities, such as lipase (LPS, A054-2-1), amylase (AMS, C016-1-1), and trypsin (TPS, A080-2-2), were assayed. Serum total protein (TP, A045-4-2), albumin (ALB, A028-1-1), globulin (GLB, E025-1-1), total cholesterol (TC, A111-1-1), total triglyceride (TG, A110-1-1), low-density lipoprotein cholesterol (LDL-C, A113-1-1), high-density lipoprotein cholesterol (HDL-C, A112-1-1), and total bile acid (TBA, E003-2-1) contents, and alkaline phosphatase (AKP, A059-2-2), lactate dehydrogenase (LDH, A020-2-2), alanine aminotransferase (ALT, C009-2-1), and aspartate aminotransferase (AST, C010-2-1) activities were determined following the guidelines offered by Nanjing Jiancheng Bioengineering Institute and Zhejiang ERKN Biotech Co.
2.5. Statistical Analyses
All of the statistical analyses reported in the figures and tables were performed using microchat v0.1.0 (
https://mineraltsai.shinyapps.io/shinymicrochat/, accessed on 22 August 2024). Data that satisfied normality (the Shapiro–Wilk normality test) and variance homogeneity (the Bartlett test of variances) were used to investigate the main and interaction effects of dietary protein and lipid inclusion levels on parameters using two-way analysis of variance (ANOVA). One-way ANOVA was employed to examine the simple effect of dietary protein inclusion level on parameters when maintaining the dietary lipid levels. Tukey’s HSD multiple comparison test was used to compare differences across groups, with ‘
p < 0.05’ indicating significant differences. The Kruskal–Wallis rank sum test was employed to assess data that did not follow a normal distribution or possess homogeneous variances, along with Dunn’s test to calculate pairwise multiple comparisons of the ranked data. Different letters in the same column represent significant differences in the main effects of all tables. For all tabulated simple effects, different letters in the same column indicate a significant effect of protein on the parameter at that level of fat fixed at a certain level.
4. Discussion
As is often the case, dietary protein presently accounts for most aquaculture costs, from which the leftover feed and undigested proteins contribute to the degradation of aquaculture waters, encouraging studies to optimize the use of dietary proteins among aquatic animals. Protein and fat serve as the major components of aquatic feeds, playing the major parts in metabolically active substance generation. Fish take in more feed to meet specific nutritional requirements as an essential nutrient becomes deficient. Protein content in feed is a key determinant of fish growth performance and feed cost [
41]. Generally, increasing protein content improves fish growth, especially in carnivorous fish [
8,
41]. In this study, FAW and WGR significantly increased and FCR significantly decreased in fish given 7% and 10% fat diets with increasing protein levels, which is consistent with the results of many species [
19,
23,
42,
43,
44]. The significantly lower protein requirement in the 4% fat group was attributed to the optimal ratio of protein to fat in the feed and the fact that hibiscus crucian carp can make good use of the fat reserves to provide protein [
43]. Earlier research reported that excess feed protein does not promote growth but increases feed costs [
45]. This is similar to previous studies where the dietary protein was reduced from 36% to 30% and the fat requirement was increased from 4% to 6% [
46,
47]. Generally, changes in HSI reflect liver function or liver size, and high HSI values are often associated with poor growth and health status [
48]. In this study, high-fat and high-protein diets significantly increased VSI and HSI, which may be due to the storage of fat in the mesentery and liver, suggesting that excessive fat and protein consumption contributed to liver damage. Similar responses were observed in
Nibea albiflora [
49] and hybrid snakeheads (
Channa maculata ♀ ×
Channa argus ♂) [
24,
41]. This was also confirmed by the higher LDH, AST, and ALT levels in the serum of Furong crucian carp given high-fat or high-protein diets. As is known to all, blood LDH, AST, and ALT activities typically reflect liver metabolic status, with higher levels indicating reduced or impaired liver dysfunction [
50,
51].
Protein efficiency ratios (PERs) and energy deposition rates (EDRs) are commonly employed to assess the protein-sparing effect of fat in feeds since protein deposition is usually influenced by non-protein energy intake [
52,
53]. In the present study, PER and EDR appeared to decrease with dietary protein inclusion levels increasing, as described in other studies on other species [
54,
55]. Although it has been shown that high-fat diets do not increase PER and EDR, and the effect of high-fat diets on protein conservation is not significant, this may be due to either total or non-protein energy deficits in the diet. Protein is used for energy supply, whereas high-fat levels and total feed energy are used for energy supply [
54]. With sufficient fat, protein is not only used for energy supply but also for protein synthesis, which makes the protein-sparing effect more pronounced at moderate-fat levels than at high-fat levels. In addition, high-fat diets are also detrimental to growth as they result in reduced food intake and nutrient inputs for growth [
56,
57]. The primary nutritional value of aquatic animals is mainly reflected in their nutrient composition [
58]. In this experiment, dietary fat and protein levels significantly affected the crude fat, crude ash, and energy of Furong crucian carp, which were significantly lower at a 35% protein level than at a 30% level, indicating that excessively high-protein and high-fat levels are not only detrimental to growth but also aggravate the metabolic burden of the aquatic animals or lead to metabolic disorders, which can lead to a decrease in their fat content. However, in the study of
Hemibagrus wyckioides, it was found that the fat level was positively proportional to the crude fat content of the fish [
54]. In contrast, in the present experiment, the crude fat content of Furong crucian carp was not significantly affected by the fat level, reflecting the fat-sparing effect on protein, indicating that feeding diets containing a fat level of 4% and a protein level of 30% were appropriate for protein utilization in Furong crucian carp, which is in line with the results of a crab study at the lipid level [
52], but the reason for the difference in protein levels may be related to the different utilization and conversion of feed nutrients by species, with crustaceans requiring higher levels of protein at the larval stage, which decreases as body weight increases.
Growth involves multiple processes that are strongly influenced by the physiology of digestion and absorption in the organism, affecting the underlying dynamics of nutrient utilization of ingested nutrients and controlling the degree of stress response in fish [
18]. The ability of fish to utilize food efficiently may depend on digestive enzymes and their response to different dietary ingredients such as proteins and fats. Feed degradation in the digestive tract of fish is largely dependent on digestive enzymes to maintain nutrient effectiveness. Adaptive changes in digestive enzyme activities concerning feed fat levels were previously reported [
59,
60]. Feed protein and/or fat content influences hepatopancreatic digestive enzyme secretion [
19]. In the present study, increased levels of feed protein/fat significantly decreased protease, lipase, and amylase activities in the gut and liver of carp given the 35%/7% group. Similar results were observed in other studies, where high protein/fat levels resulted in a low activity of digestive enzymes [
41,
60,
61]. As information on the effects of dietary protein and fat effectiveness on foregut histomorphometry is limited, further studies are needed.
Serum TC and TG levels reflect the body’s ability to utilize protein and fat. In addition, in high-protein fish diets, protein content exceeds requirements. Excess ammonia produced by metabolism may interfere with serum ion concentration balance and oxygen consumption, increasing the metabolic cost of excretion and reducing growth [
62]. High-fat diets may also reduce growth because the body’s ability to utilize high fats is limited. Excess fat is stored in the hepatopancreas or other organs, leading to metabolic imbalances, or the high-fat diets themselves are nutritionally unbalanced and do not meet the body’s growth needs [
63]. On the contrary, in the present study, changes in hematological parameters were observed concerning the protein and fat levels in the feeds, with significant differences in the hematological parameters due to the different fats in the feeds. The serum TC level increased as the feed fat level increased from 4% to 8%, and an increase in TG levels was always accompanied by an increase in TC [
64,
65], while serum TC and TG levels decreased as the feed protein level increased. This suggests that endogenous fat consumption is active, which is similar to the studies on toothfish (
Paralichthys olivaceus) [
66] and yellow goby (
Nibea diacanthus) [
67]. Furthermore, dietary proteins play an important role in immune responses [
34,
35], as evidenced by the changes in serum ALB and GLB levels in fish given high-fat diets. AKP serves as an important regulatory factor in the innate immune system of animals [
42], which can catalyze the hydrolysis of phosphate monoesters and the transfer reaction of phosphate groups and have an indispensable role in non-specific immunity. In this experiment, blood AKP was not significantly affected by protein levels, fat levels, or their interaction. The reason for the differences may be due to the interaction between fat and protein levels in this experiment, and differences in species and feed formulations. The mechanism of action of the interaction between protein and fat levels in crustaceans and fish also differs, which requires further research.