1. Introduction
Sugarcane is an important economic crop around the world and is used to produce sugar, ethanol, bagasse, molasses, and animal feed. The top four producers are Brazil, India, China, and Thailand, with Thailand ranking second in global sugar exports, behind Brazil [
1]. In 2020, world agriculture produced 181.1 million tons of sugar, with 8.4 million tons from Thailand alone [
2]. Drought significantly impacts sugarcane yield, reducing it by up to 60%, particularly during the formative stage [
3,
4]. In the late rainy season production system used in northeast Thailand, drought often occurs at two developmental stages: (1) the tillering phase, which experiences water stress during the dry season, and (2) the elongation stage, which faces intermittent drought during the rainy season [
5].
Drought stress significantly negatively impacts sugarcane growth, physiology, and yield. Breeding drought-resistant cultivars has become a crucial strategy, requiring a deep understanding of drought-resistant mechanisms. Sugarcane is particularly susceptible to water stress during the tillering and stem elongation periods because both growth stages have high water requirements [
3,
6]. The importance of drought-tolerant sugarcane in global agriculture is becoming increasingly critical, due to the growing challenges posed by climate change and water scarcity. The development of drought-tolerant sugarcane is important as it enhances yields in drought-prone areas and provides a sustainable solution [
7,
8]. Drought affects the early stages of sugarcane physiology [
8]. The basic mechanisms that drought-resistant sugarcane employs to resist water stress involve various physiological and agronomic attributes throughout the initial stages of growth [
9]. The physiological traits and root system of sugarcane adapt to water stress during the early growth stage [
10]. Drought stress reduces the leaf chlorophyll content, which is more pronounced in drought-sensitive cultivars [
11]. Leaf rolling, stomatal closure, the inhibition of stalk and leaf growth, leaf senescence, and reduced leaf area are common drought stress responses in sugarcane [
6].
Drought stress at early growth stages significantly decreases the PSII efficiency, SCMR, leaf water status, and stomatal conductance [
3,
8,
12,
13,
14]. Stomatal limitations, which affect stomatal conductance (Gs), transpiration rate (E), and internal CO
2 concentration (Ci), are the main variables contributing to a reduction in photosynthesis in sugarcane [
7,
15,
16,
17,
18]. The drought-resistant sugarcane cultivar shows strong adaptation in water use efficiency under limited water conditions [
19]. Drought-tolerant cultivars exhibit higher stomatal density, deeper root systems, and better osmotic adjustment compared to drought-susceptible ones [
6]. Proline functions as an osmolyte, helping sugarcane plants maintain water balance and cell turgor by adjusting the osmotic potential [
20]. Its accumulation is linked to enhanced photosynthesis, growth, and overall drought tolerance [
21]. Evaluating proline levels is an effective method for identifying drought-resistant sugarcane genotypes [
22]. Drought stress affects physiological processes, which in turn impact growth, particularly in terms of number, height, stalk diameter, and stalk weight [
3]. Water stress disrupts cell division and elongation, with stem and leaf growth being the most affected [
23]. Root development is also impacted, though to a lesser extent than above-ground biomass [
6,
24]. The size and distribution of the root system, along with the physiological responses, are directly related to sugarcane’s yield productivity under early-season water stress [
24,
25]. Drought-resistant sugarcane cultivars maintain high leaf water status by minimizing water loss and optimizing root water uptake [
10]. These cultivars sustain a high proportion of root and shoot biomass even under water deficit conditions, ensuring an adequate water supply to above-ground plant parts [
17]. However, in previous studies, biomass was drastically reduced by 49.7% during the stalk elongation phase, where dehydration significantly hindered stalk growth [
22,
23].
Physiological parameters and agronomic traits can be readily measured and used as key criteria for selecting drought-tolerant sugarcane in its formative stages [
3]. Indicators of drought tolerance include high chlorophyll fluorescence, chlorophyll content, stomatal conductance, and relative water content [
8]. Key agronomic traits for drought resistance include high biomass, adaptability, good root distribution, and high sugarcane yield [
26,
27]. Despite extensive research, information on the specific effects of drought during the tillering and stalk elongation stages in the late rainy season of northeast Thailand is still lacking. This study aimed to explore the responses of diverse sugarcane genotypes grown under water-withholding conditions during these critical developmental stages. The findings will provide valuable insights for developing drought-resistant varieties and enhancing sustainable sugarcane production.
3. Discussion
Several important physiological and biochemical traits influence the sensitivity of sugarcane to drought during its formative stages [
28]. Sugarcane exhibits drought tolerance characteristics in response to slight to moderate droughts through drought tolerance mechanisms; however, under severe drought conditions, it employs drought avoidance mechanisms [
6]. Each genotype exhibits different responses to water stress. During the tillering stage, KK3 showed decreased Gs, Fv/Fm, SCMR, and RWC in response to water-withholding conditions, which is consistent with the findings of Leanasawat et al. (2021), who reported significant reductions in the maximum quantum yield of PSII efficiency and stomatal conductance under water stress. The reduced Gs and Fv/Fm in KK3 led to decreased height and biomass compared to well-watered conditions [
10]. Despite low SCMR, Gs, Fv/Fm, and RWC values, KK3 maintained good yields under drought conditions [
25]. K88-92 exhibited a similar physiological response to KK3. However, the authors of Khonghintaisong et al. (2018) found that K88-92 did not exhibit significant changes in Gs and Fv/Fm under stress. The authors of Songsri et al. (2019) reported strong SCMR values for K88-92 in drought conditions, along with high proline content in the roots, indicating a drought-tolerant genotype. Drought-tolerant varieties accumulate high levels of proline [
20]. UT12 is sensitive to drought, as evidenced by its low Gs, Fv/Fm, SCMR, and RWC values, resulting in reduced biomass and yield [
7,
25]. MPT14-618 showed decreased physiological traits in response to water-withholding treatment but exhibited the smallest decrease in biomass. Drought tolerance was exhibited by genotypes that maintained their ability to produce biomass despite decreased gas exchange [
8]. MPT14-618 showed higher proline accumulation in its leaves compared to other genotypes, which aligned with the finding that proline accumulation is higher in drought-tolerant varieties [
12]. PK4 exhibited similar physiological responses to UT12.
For the drought-resistant genotype MPT14-618 used in this study, during the tillering phase, the minimal reduction in biomass under water-withholding conditions can be attributed to acclimation mechanisms such as lower Gs, which reduces water loss by transpiration, and increased proline production under drought conditions. In addition, there was a good recovery of Fv/Fm during the re-watering period. However, another drought-resistant genotype in this study, KK3, exhibited minimal reductions in RWC, Fv/Fm, and SCMR, indicating that these traits play a key role in drought resistance during the tillering phase. In contrast, UT12, which demonstrated susceptibility to drought in this study, did not exhibit any traits that support drought resistance during the tillering phase. During the elongation stage, KK3 and K88-92 appear to rely on RWC responses to resist water deficit, while MPT14-618 utilizes Gs acclimation. Each genotype clusters differently based on these primary and secondary traits, indicating variations in their response or recovery dynamics under early-season drought stress (
Figure A2).
After re-watering, the growth of KK3 was rapid, resulting in no difference in shoot dry weight compared to the control, and the biomass of K88-92 was identical to that of the control [
10]. UT12 exhibited reduced cane yields with decreased SCMR, Fv/Fm, RWC, and stomatal conductance during both the drought stress and recovery periods [
25]. Tolerant varieties quickly returned to their control values after recovery, while susceptible varieties failed to do so [
12]. During the stalk elongation stage, KK3, K88-92, and MPT14-618 displayed comparable physiological reactions, with decreased SCMR, Gs, Fv/Fm, and RWC, although this was less pronounced than in the tillering stage. Most sugarcane production areas in Thailand are rain-fed, and droughts typically occur during the growing season, especially in the early stages [
10]. The authors of Chapae et al. (2020) also observed that water deficits were more apparent early in the growth stages but not during stalk elongation. Consequently, the biomass of these three genotypes under WW treatment was similar to FC conditions. Drought stress reduced cane yield, but KK3 outperformed other genotypes in cane and sugar output, demonstrating resilience and recovery ability after re-watering [
9]. In contrast, UT12 and PK4 had reduced biomass under drought conditions, due to significant physiological decline. The root dry weight of UT12 decreased under drought conditions, while KK3 remained unaffected [
7]. KK3 has better water absorption than UT12 because it had a higher percentage of xylem vessel area over stele area, while the root volume, surface area, and length of KK3 and UT12 were similar. Furthermore, KK3 exhibited greater average stomatal density and stomatal dispersion in comparison to UT12 [
18].
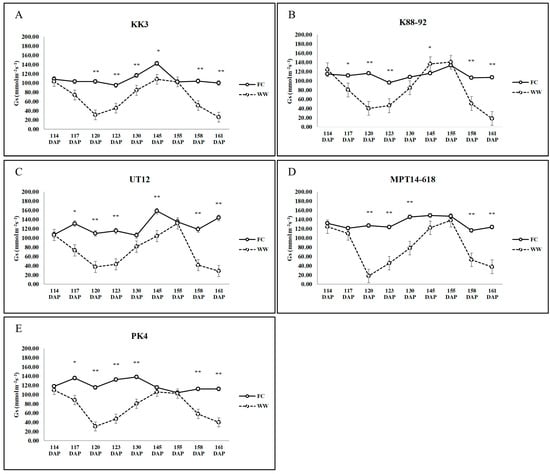
Research indicates that sugarcane employs a combination of drought avoidance and tolerance mechanisms to mitigate the effects of water stress. By reducing transpiration, accumulating osmotic solutes, maintaining photosynthetic efficiency, and enhancing water uptake through its root system, sugarcane can effectively survive and even recover from drought conditions. These mechanisms vary among different genotypes, with some exhibiting stronger drought resistance than others. One of the key responses to drought stress in sugarcane is a reduction in transpiration. The closure of stomata (lower Gs) reduces water loss through transpiration, thereby conserving water in the plant. This drought avoidance mechanism helps maintain hydration within the plant tissues during periods of water shortage [
6,
29]. By reducing transpiration, sugarcane can conserve water while continuing to engage in those metabolic processes essential for survival. After three days without water availability, every variety displayed decreased Gs and significant differences (
Figure 1), and showed reduced RWC (
Figure 4). Maintaining photosynthetic activity during drought stress is another critical strategy for sugarcane survival. The reduction in leaf water content, as seen in the reduced RWC and chlorophyll content, is often associated with decreased photosynthetic efficiency. However, drought-tolerant genotypes like KK3 and MPT14-618 showed good recovery of Fv/Fm after re-watering, indicating that they are capable of sustaining photosynthesis despite initial reductions in RWC. This capacity for photosynthetic maintenance under water stress is crucial for ensuring continued energy production, which supports vital growth processes such as biomass accumulation [
9]. Drought tolerance is the plant’s ability to grow and produce biomass in low-water environments [
29]. There was a significant variation in plant height between the two water treatments and formative stages; plants undergoing the well-watered treatment were significantly taller than those undergoing the drought stress treatment [
28]. However, the MPT14-618 variety consistently demonstrated increased height during both the water and growth stages (
Figure 6) and showed a small percentage of biomass reduction (
Figure 8A,B). In addition, sugarcane also utilizes a mechanism to maintain cell turgidity through osmotic adjustment. This involves the accumulation of solutes such as proline, which lowers the osmotic potential in plant cells, facilitating water absorption from the soil even under drought conditions. Plants lower their osmotic potential, allowing the roots to absorb water and maintain cell turgidity, enabling survival in arid conditions through higher leaf chlorophyll content, increased stomatal conductance, sustained photosynthesis, growth maintenance, and osmotic adjustment [
6]. Higher proline content was observed in drought-tolerant varieties like K88-92 and MPT14-618, especially in their roots, highlighting the importance of osmotic adjustment for maintaining water uptake and cell hydration during water stress. The ability to adjust osmotic potential enables these varieties to survive and grow, even in low-water environments, by maintaining cell turgidity, sustaining photosynthetic processes, and ensuring growth maintenance despite reduced water availability [
6,
29]. Proline accumulation in sugarcane roots indicates an osmotic adjustment, with higher levels of proline observed under drought conditions, suggesting a positive correlation between proline accumulation and drought tolerance [
30,
31,
32]. Understanding these mechanisms is crucial for breeding programs aimed at developing sugarcane varieties that are resilient to drought, particularly in regions where water availability is unpredictable.
4. Materials and Methods
4.1. Plant Materials and Experimental Details
The experiment was conducted under close greenhouse conditions from October 2021 to May 2022 at the Mitr Phol Cane and Sugar Research and Development Co., Ltd., Chaiyaphom, Thailand (16.4713° N and 102.1246° E). A 2 × 5 factorial experiment in a completely randomized design with four replications was used. Factor A consisted of two water regimens: (1) soil moisture, maintained at field capacity throughout the experiment (no water-withholding treatment; FC) and (2) short water-withholding conditions during the tillering and elongation stages (water-withholding treatment; WW). During the tillering stage, water was withheld from 114 to 120 days after planting (DAP), and then the plants were re-watered. Water was withheld again during the stalk elongation stage, from 155 to 161 DAP. Factor B included five sugarcane varieties with varying drought resistance levels: KK3 and K88-92 (drought-resistant and high-yielding in the late rainy season), UT12 (drought-susceptible and recommended for irrigation systems), MPT14-12-618 and PK4 (high-yielding in the late rainy season).
The experimental water regimes were designed based on the drought periods experienced during the late rainy season in northeast Thailand. Drought commonly occurs at two developmental stages in sugarcane production: (1) the tillering phase, with plants facing water stress during the dry season, and (2) the elongation stage, with plants encountering intermittent drought during the rainy season. KK3, a drought-resistant and high-yielding variety popular in Thailand, was included in the study. K88-92 served as a drought-resistant check, while UT12 was used as a drought-susceptible check. MPT14-12-618 and PK4, notable high-yielding varieties from the Research and Development Innovation Center at Mitr Phol Cane and Sugar Research and Development Co., Ltd., were also included.
Each pot, with a volume of 18 cubic meters and containing 20 kg of dry soil, was used for planting. The soil texture was a sandy loam with a pH of 6.70. Organic matter (OM) was 0.74%; electrical conductivity (EC) was 0.13 ds m−1; total nitrogen was 0.043%; available phosphorus was 58.75 mg kg−1; exchangeable potassium was 152.24 mg·kg−1, and the bulk density was 1.44 g cm−3. Healthy sugarcane seedlings, free from disease and pests, were initially grown in trays for 30 days to ensure uniform germination. Selected uniform seedlings were then transplanted into pots, with one seedling per pot. A basal fertilizer of N-P2O5-K2O was applied at rates of 50 kg ha−1, 50 kg ha−1, and 25 kg ha−1, respectively. Monthly applications of a fertilizer made from N-P2O5-K2O were applied at rates of 65.63 kg ha−1, 21.88 kg ha−1, and 56.25 kg ha−1, respectively. Applications at the same rates were made from the first month after transplanting until the end of the experiment.
4.2. Meteorological Conditions and Soil Moisture Content
Meteorological data were collected daily throughout the experimental period, from October 2021 to May 2022. The average temperature ranged from 17.2 to 31.5 °C (
Figure 9A), and the relative humidity ranged from 55% to 92%. During the experiment, a total of 550.62 mm of rain was recorded (
Figure 9A). There was 15 mm of rainfall during the stalk elongation stage, but none fell during the tillering stage. Even though there was rainfall in natural outdoor conditions, this experiment was conducted in a closed-roof greenhouse, which remained unaffected by the rainfall throughout the study.
Soil moisture content was measured at 3-day intervals from 90 DAP to 161 DAP. Field capacity (FC) was 10.92%, and the permanent wilting point was 4.69%. During water-withholding treatment, soil moisture contents varied between the different water regimes. At the tillering stage, the soil moisture content was 5.16% under water-withholding conditions at 117 DAP and 3.50% at 120 DAP. At the stalk elongation stage, it decreased to 4.67% at 158 DAP and 3.39% at 161 DAP (
Figure 9B).
4.3. Physiological Traits
The physiological traits measured included chlorophyll fluorescence (Fv/Fm), SPAD chlorophyll meter reading (SCMR), stomatal conductance (Gs), and relative water content (RWC). Chlorophyll fluorescence was measured between 8:30 and 10:00 a.m. using a Handy-PEA (Hansatech Instruments, UK) on fully expanded leaves, in the middle of the second leaf. SPAD chlorophyll content was measured between 8:30 and 10:00 a.m. using a SPAD-502 Plus (Konica Minolta, Japan). Stomatal conductance was measured between 10:00 and 12:00 a.m. using a Dynamic AP4 (Delta-T Devices, UK). Relative water content, which is a trait that is calculated by destructive sampling, was measured when collecting the same leaves as for other characteristics, between 10.00 and 11.00 am. The leaf blade samples were cut into leaf discs (1 cm
2 each) and were used to determine RWC. Once in the laboratory, the fresh mass was recorded and the discs were immersed in distilled water for 24 h, then blotted dry and reweighed to obtain the water-saturated mass. The dry mass of the discs was recorded after drying at 80 °C for 48 h. RWC was calculated using the following equation:
4.4. Proline Content
At 120 DAP, during the water-withholding period, samples were collected from the fully expanded fourth leaf of the main shoot and from the middle of the root system. Both leaf and root samples were ground in liquid nitrogen and weighed to 250 mg (fresh weight). Proline was then extracted and quantified using a spectrophotometer at 520 nm to determine the total proline content. The calculation formula is as follows:
4.5. Growth Traits
At the tillering stage, cane height was measured from 90 to 120 DAP, and the number of shoots was counted before and during the water-withholding period. Biomass (root, shoot, and leaf dry weight) was collected at 120 DAP. At the elongation stage, cane height was measured from 123 to 161 DAP, and the number of stalks was counted before and during the water-withholding period. Biomass (root, shoot, and leaf dry weight) was collected at 161 DAP.
4.6. Data Analysis
The variance for all traits was analyzed using a factorial experiment in a completely randomized design (CRD), and mean comparisons were performed using the least significant difference (LSD) method with the Statistix 10 software program (Analytical Software, Tallahassee, FL, USA).
5. Conclusions
Drought impacts the physiological characteristics of sugarcane during both the tillering and stalk elongation stages, with the tillering stage being more severely affected. This reduction in physiological traits hinders overall growth. Genotypes that adapt well during drought periods in the tillering stage can better cope with subsequent drought periods in the elongation stage. KK3, K88-92, MPT14-618, and PK4 showed similar physiological responses to drought, but this was in contrast to UT12. The most notable changes were observed in RWC and Gs, with genotypes like KK3 and PK4 demonstrating better drought tolerance. Proline contents in the roots of K88-92, KK3, and MPT14-618 increased under drought conditions, indicating osmotic adjustment and enhanced water absorption capability. Consequently, these genotypes maintained biomass levels similar to those in normal water conditions. Biomass production varied significantly, with MPT14-618 exhibiting the least reduction. KK3, MPT14-618, and K88-92 maintained biomass levels better under drought conditions compared to UT12 and PK4, which showed the highest sensitivity to drought. The findings indicate that drought stress impacts sugarcane genotypes differently, with KK3, K88-92, and MPT14-618 showing better physiological and growth resistance. These genotypes also tend to produce high yields despite drought conditions. Furthermore, understanding the drought responses of sugarcane genotypes will be crucial for characterizing new varieties under intermittent drought conditions during the early growth stage, providing valuable insights for breeding programs. To accurately assess the response to intermittent drought stress during the early growth stage of sugarcane, it is recommended that field trials under real-world conditions should be established, as they more effectively replicate natural environmental variables.