1. Introduction
Each year, approximately 50 million tons of lignin are generated globally, with 98% to 99% incinerated for the purpose of producing energy and steam in pulp manufacturing facilities. Only a fraction, primarily, is commercially recovered under a biorefinery concept. There are significant opportunities to create high-value products from technical lignin, especially given the versatility of these modified materials in the catalysis industry [
1,
2]. Generally, when organic matter composed of hemicellulose, cellulose, and lignin is subjected to thermochemical processes such as pyrolysis, carbonaceous materials are generated, characterized by aromatic structures, and oxygenated groups, including aldehydes, ketones, esters, and carboxylic acids. These characteristics endow these materials with the capacity to adsorb molecules classified as emerging contaminants and heavy metals from water through electrostatic interactions, van der Waals forces, and π-stacking, making them an excellent alternative in addressing current environmental challenges [
3,
4,
5]. This characteristic allows for the reuse of the carbonaceous material and contributes to addressing an environmental issue, such as the persistence of organic molecules and heavy metals in wastewater and drinking water. However, contaminants merely move from one matrix to another, and measurements must be implemented to prevent their accumulation. An important strategy is utilizing these pyrolyzed materials as carbon catalysts [
6,
7].
To address emerging environmental issues of the 21st century, unmarketable potato peels can be further processed and transformed into value-added materials [
8]. Potatoes are among the most significant agricultural crops for human consumption, following wheat, rice, and maize, with a production volume of 376 million tons in 2013 [
9]. However, the potato industry generates substantial waste—leftover tubers, peels, and raw materials—that accumulate and pose environmental challenges. It is estimated that between 12% and 20% of the total volume of potatoes processed results in peelings and other by-products [
10]. Similarly, bananas are widely cultivated and nutritionally valuable crops, grown across more than 130 tropical and subtropical countries. In 2016, India, China, the Philippines, Brazil, and Ecuador were the leading banana producers, with a global yield of approximately 144 million metric tons, highlighting its economic relevance [
11]. Despite this, a large portion of the banana plant is discarded as waste, disrupting ecosystems and contributing to ecological hazards. Banana pseudostems, often seen as low-value waste, can also be repurposed into valuable materials, further supporting efforts to mitigate contemporary environmental challenges [
12].
Both species represent high-value agroindustrial waste, however, their application in adsorption and/or biocatalytic processes can be influenced by the generation of new physicochemical properties from the pyrolysis process and its lignitic composition. The composition of these residues is shown in
Table 1 [
13,
14].
The characteristics resulting from the pyrolysis process in both species can result in the generation of molecules in a greater or lesser proportion, directly influencing their applications. For example, the greater amount of lignin in banana pseudostem results in a greater production of phenols and aromatic hydrocarbons, which are byproducts of lignin decomposition [
15]. Currently, several studies have developed similar materials with environmental applications. An example is the work of Grisales et al. [
16], who produced biochar derived from palm fiber residues, in combination with H
2O
2 and O
5S
−2, in the removal of drugs such as valsartan, acetaminophen, and cephalexin in water. Similarly, Quimbaya et al. [
17] produced a carbonaceous material derived from industrial waste, specifically sawdust, which was modified with manganese to form an improved biochar. This material was used to activate PMS by ultrasound (US) at different frequencies, achieving the degradation of ciprofloxacin in water. Demonstrating that biochars can be optimized by modifications with transition metals and whose improvement potential depends on the molecular composition of the species in their cell walls, in addition to these methods, the transcendental value and carbocatalytic power of the waste are demonstrated even by incorporating an oxidizing agent in the system.
In this context, it is necessary to add oxidizing agents to the carbonaceous material that are activated by electron transfer, allowing the decomposition and even elimination of the contaminant. Thus, various catalytic carbons have been synthesized that activate inorganic peroxides (hydrogen peroxide, peroxymonosulfate, and peroxydisulfate), generating radical species (
•HO,
•SO
4−) and non-radical species (
1O
2) after the homolytic bond dissociation of the peroxide (O-O) bond. These species are capable of degrading persistent molecules such as antibiotics (β-lactam antimicrobials, sulfa drugs, 4-aminobenzoic acid, and tetracycline-class compounds) and antipyretics like acetaminophen, among others [
18], Equations (1)–(6).
ACE is classified as an emerging contaminant. These are chemical substances environmentally poorly regulated, with adverse effects on human health, which are not fully known. Personal care products, pharmaceutically active compounds, and endocrine-disrupting chemicals are among the most popular CECs. Many CECs are bio-accumulable and recalcitrant to conventional processes in wastewater or drinking water. The ACE can induce adverse physiological effects at low concentrations in humans and animals. Numerous studies worldwide have reported its presence in various aquatic environments (200 ng/L), raising concerns about the potential negative impacts on water quality, human health, and wildlife. The ACE generates intermediates depending on pH. Its degradation products (hydroquinone and benzoquinone, among others) and intermediates present significant challenges for effective monitoring, detection, and the development of suitable treatment technologies. Membrane separation and third treatments as oxidation advance the process are options to contribute to this problem [
19,
20]. Various materials have been developed from carbon-based substances for the degradation of ACE, frequently incorporating nitrogen- and phosphorus-doped carbon nitrides activated through photocatalysis [
21,
22]. Furthermore, oxidizing agents like periodate and peroxydisulfate have also been utilized. Reactive oxygen species have been produced in every instance, resulting in significant acetaminophen degradation rates. However, there is limited research on the role of transition metals in cleaving the peroxide bond within a carbonaceous matrix, particularly when carbonate ions are included in the process. These ions enhance surface area and generate carbonate radicals, which act as additional active species for the oxidation of organic compounds,
Table 2.
On the other hand, an advantage of the PMS is its ease of forming coordination bonds with transition metals, which in turn bond with the carbonaceous matrix, facilitating the cleavage of the double bond. On the other hand, due to its high redox potential, it allows it to oxidize metal oxides, which in turn performs a direct oxidation to the contaminant. Equations (7)–(9).
Considering that PMS can be activated by carbonaceous matrices and transition metals, it is expected that the combined effect of these two factors improves performance in removing recalcitrant molecules from aqueous solutions. In this study, banana pseudo-stem, and potato peel were used as sources of lignocellulosic materials. These materials were then thermally exfoliated at 500 °C and subsequently modified with manganese to create a carbon catalyst capable of completely degrading acetaminophen. The nature of raw carbonaceous materials was evaluated to comprehend its implications in the synergy phenomena for the breakdown of organic pollutants. Finally, to demonstrate how lignocellulosic waste can be used to activate PMS. The role of transition metals and their carbonate salts in the process, as well as the structural implications for degrading ACE via reactive oxygen species. This is shown using electrochemical techniques to evidence the degradation and the material’s capacity to transfer electrons.
4. Discussion
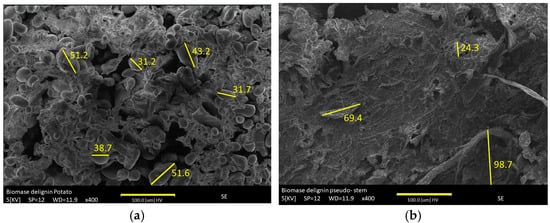
In
Figure 2a–d the morphological change of the raw material compared to the modified material is observed. The mesoporosity increases due to temperature. The gases generated during the pyrolysis process promote thermal exfoliation. However, it is notable that the metal decreases mesoporosity due to an increase in its width and volume (
Table 3). This may be related to the ease with which the carbonate in the metal salt generates CO
2 and its effect on the system’s exfoliation. The CO
2 generated from the carbonate stabilizes metal oxides, which increases the surface area (Equation (11)), but also fragments the particle and contributes to the stability of metal oxides. The surface manganese content is displayed, highlighting its effect on increased nucleation in the carbon matrix. The manganese is homogeneously distributed in both functionalized materials for BPP-Mn and BPS-Mn.
Figure 3a–d,
Table 4 [
32].
In
Figure 4, two amorphous halo patterns of the cellulose are observed with a wide band around 2θ = 25°, characteristic of activated carbon. For BPS, a peak at 29.5° is observed, which can be related to the existence of inorganic components including SiO
2. The presence of manganese carbonate and surface Mn oxide is evidenced in the diffraction patterns, confirming the thermal effect on the formation of oxides and crystallization of the excess metal salt on the surface for BPS-Mn. Alternatively, in the presence of adsorbed surface water, these systems can promote hydroxide formation on the material’s surface (Equation (12)) due to the influence of carbonate in the structure. This leads to the negative surface charge residual, as indicated in
Table 4.
Figure 5a,b shows the adsorption and degradation processes of all materials concerning ACE. The pyrolyzed BPS material exhibited a synergistic effect, functioning as a carbocatalyst. However, the incorporation of metal ions into the system enhanced the adsorption of ACE. The electrochemical behavior, represented by the oxidation peak associated with manganese, was inhibited by ACE, suggesting the formation of a stabilized coordination compound, which in turn reduced the carbocatalytic activity. Lignin is the strongest component due to its highly aromatic structure of phenylpropane units in BPS concerning BPP as reported in the literature and observed in electrochemical analyses, evidencing vanillin. When lignin is pyrolyzed, the β-O-4 bonds break, forming a series of free radicals, which repolymerize to produce bio-oil, syngas, and biochar. To 500 °C phenolic compounds such as vanillin favor the coordination bonds between carbonaceous material-Mn:PMS: ACE, in high grade concerning materials with low lignin content as potato peel [
33]
Figure 6a–c,e–g. On the other hand, the carbonate/bicarbonate ions are strong scavengers of both hydroxyl and sulfate radicals, with high reaction rate constants, which may explain this effect, low carbocatalysis in the presence of metal carbonate (Equations (13) and (14)) [
34] as depicted in
Figure 6d,h.
Table 5 shows that only the treatment carried out with the most active material (BPS) for degradation exhibits an increase in pH after treatment. This behavior suggests heterolytic cleavage of peroxide bond in PMS, activated by transfer electronic since carbonaceous material, Equation (15) [
25].
On the other hand,
Figure 8a confirms the formation of sulfate or hydroxyl radicals during the carbocatalytic process for BPS when methanol is employed as a scavenger. However,
radicals also contribute to in breaking down ACE. It is suggested that BPS is reduced through interaction with PMS, as shown in Equation (16). This happens because PMS is activated by the carbon-based material in BPS, due to its ability to transfer energy. The impedance study, shown in
Figure 8b, supports this by highlighting the role of oxidation products of lignin [
35]. The RAMAN spectra (
Figure 6c) show the use of sp2 hybridized active sites, which aid the breakdown process after treatment.
As illustrated in
Scheme 1, the proposed mechanism for ACE removal involves three key contributions: (1) the role of manganese in BPS, which aids in removal through adsorption, and (2) removal via degradation. The manganese, in forms like MnO or MnCO
3, is supported by the carbon matrix, facilitating the adsorption of acetaminophen by forming a coordination complex between the carbon matrix, Mn, PMS, and ACE. This reduces the carbocatalytic process in BPS and minimizes the contribution to peroxo bond cleavage, while carbonate ions act as radical scavengers. In contrast, pyrolyzed BPS materials without manganese (3) show that the carbon matrix itself significantly affects drug removal, possibly due to the presence of phenolic groups (lignin derivates) that persist at 500 °C, enabling the electron transfer process to PMS. It also shows a blockage in the carbocatalysis process due to the cleavage of the peroxo bond in PMS assisted by the transition metal. This is mainly because the electrochemical profile lacks oxidation peaks of new manganese species that would confirm this degradation pathway. However, it is highlighted that BPS with manganese reduces its carbocatalytic capacity, possibly due to the presence of carbonate ions acting as scavengers of hydroxyl and sulfate radicals [
36,
37,
38].
On other hand, previous studies have reported that the incorporation of transition metals, such as manganese and iron, onto carbonaceous materials enables the development of adsorbent and catalysts enhanced activity [
39,
40]. The metal oxide has also been employed to improve the adsorption characteristics of biochar. The biochar enriched with MnO
2 displayed significantly greater adsorption capacity for methylene blue dye compared to the unmodified biochar. The maximum reported adsorption capacity was 248.96 mg/g, with a removal rate of 99.73%, in contrast to 234.65 mg/g and 94.0% for the unaltered biochar [
41]. For catalytic systems, the metal enables additional active sites for redox reactions and the promotion of electron transfer between the catalyst and reactants, which facilitates the efficient activation of PMS and the generation of ROS for oxidation reactions [
42]. Researches evaluated reduced graphene with Mn and Fe
2O
4 in increased degradation of pollutants and PMS activation (process-denominated carbocatalysis). Furthermore, the adsorption process with MnFe
2O
4 and MnFe
2O
4-graphene reached ~10% of orange II removal. The transition metal oxides as the manganese (Mn) can activate oxidants [
43,
44]. However, Mn is not found as a metal but as oxides, silicates, and carbonates [
45]. Among them, the manganese oxides (MnO
X) have attracted great interest because low toxicity, various forms with various oxidation states (II, III, IV, and VII), and their capacity for PMS or PDS activation in a heterogeneous system [
46,
47]. The literature has reported that MnO
2 [
48], Mn
2O
3 [
49,
50], and Mn
3O
4 can activate PMS and PDS due to redox properties between different valence states of Mn. Among the mentioned oxides MnO
2 is the manganese oxide most studied including those amorphous and crystalline structures (α-, γ-, β-, and δ-MnO
2). Mn-containing catalysts include direct oxidation of compounds, electron transfer, pollutants oxidation by free radicals (SO
4•−, HO
•, O
2•−) [
46], and non-radical by singlet oxygen (
1O
2) oxidation. As reported, there are few cases where manganese is incorporated into the structure as carbonate. Likewise, the implications of using the carbonaceous matrix as a support or as a source of oxygenated groups available for coordination and activation of the peroxide bond are not widely explored.