1. Introduction
Bacteria are everywhere in our lives, but pathogenic strains cause infectious diseases, epidemics and deaths in cases where the tracking and classification cannot be done accurately, quickly, easily, affordably and on-site. The development of rapid and sensitive methods for the effective detection of
E. coli is important not only for patients but also for public health. Inadequate detection of
E. coli results in prolonged treatment time and thus the spread and worsening of infectious diseases [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12].
Conventional detection techniques currently utilized for
E. coli detection consist of culture (plating and colony counting) methods, polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA) [
2,
3,
7,
11,
12]. All of the traditional methods mentioned are too slow for the timely identification of an epidemic or bioterrorism attack response [
13,
14].
Nowadays, nanotechnology-supported optical measurement-based methods are in demand as they provide cheaper, safer, faster and more sensitive results [
15]. Although fluorescent [
16] (Limit of Detection (LOD): 8.3 × 10
3 CFU/mL), colorimetric [
17] (LOD: 10
4 CFU/mL), surface-enhanced Raman spectroscopy [
18] (LOD: 10
5 CFU/mL), surface plasmon resonance [
19] (LOD: 1.1 × 10
6 CFU/mL) and chemiluminescence [
20] (LOD: 10
3 CFU/mL) methods are mostly preferred in optical detection, smartphone-connected, paper sensor-based sensors also come to the fore due to their advantages of low cost, portability and short detection time [
21,
22,
23,
24].
Another platform that stands out in bacterial detection is electrochemical sensors whose electrodes are modified with nanomaterials [
25]. In particular, magnetic nanoparticle-based electrochemical sensors provide results with LODs as low as 1 × 10
2 CFU/mL [
26], 10 CFU/mL [
27] and 2.84 × 10
3 CFU/mL [
28], respectively. In their review study, Štukovnik et al. (2023) stated that nanomaterial-supported impedimetric electrochemical biosensors have high selectivity, sensitivity and response time [
29]. In a study combining the advantages of magnetic nanoparticles and impedimetric electrochemical sensors, the authors developed a sensitive, rapid and cost-effective assay for the detection of
Escherichia coli (E. coli) in drinking water and apple juice [
30]. The detection is based on electrical impedance spectroscopy measurements with screen-printed interdigitated electrodes combined with magnetic nanoparticles functionalized with the antimicrobial peptide melittin (MLT). With this approach, it was possible to detect
E. coli concentrations of up to 1 CFU/mL in drinking water and 3.5 CFU/mL in apple juice with a linear range of 1–10
6 CFU/mL in just 25 min without sample preparation. The disadvantages of the study are the high cost of sensor production, the need to process the impedance spectroscopy data with multidimensional projection techniques, and the time spent on the processes performed.
The popularity of quantum dots as nanomaterials in impedimetric biosensors has increased thanks to their distinctive qualities such as electrocatalytic activity, tunable size, good signal amplification and multiplex detection capability [
31]. In the study reported by Zong et al. (2019), they utilized CdS quantum dots-encapsulated metal-organic frameworks as signal-amplifying tags and were able to detect
E. coli at a sensitivity limit of 3 CFU/mL [
32]. In the same study, they stated that the use of quantum dots has promising advantages in terms of sensitivity and selectivity in electrochemical immunosensor applications.
However, the literature is still lacking in a biosensor that can measure with high precision in a short time, which does not take long to prepare for the sensor, can enable optical and electrochemical measurement methods, and can do all these at low cost. Therefore, to overcome the transducer handicaps mentioned above with its fast and specific sensing capability, low cost and high sensitivity, our hybrid biosensor study combines a paper-based electrochemical biosensor and magnetic quantum dots (MQDs) in a novel approach. Our proposed biosensor has remarkable advantages as follows: (i) the sensing system is capable of taking measurements with high precision in 30 min, (ii) the sensor can be achieved using stock magnetic quantum dots within 90 min, (iii) the system utilizes low-cost materials such as paper and pencil, (iv) the system is capable of multi-detection with simultaneous electrochemical and optical sensing.
2. Materials and Methods
2.1. Materials
Cadmium oxide (CdO, 99.99%), zinc acetate (ZnAc, 99.99%), selenium (Se, 99.99% powder), sulfur (S, 99.998% trace metals basis), oleic acid (OA, 99.99% technical grade), 1-octadecene (1-ODE, 90% technical grade), trioctylphosphine (TOP, 97% technical grade), iron(0)pentacarbonyl (Fe(CO)5, >99.99% trace metals basis), oleylamine (OAm), Trimethylamine N-oxide (TMNO, 95%), N-hydroxysulfosuccinimide (Sulfo-NHS), N-(3-dimethylaminopropyl)-N′-ehylcarbodiimide hydrochloride (EDC), pyridine, Hexane (anhydrous, 95%), ethanol (EtOH, 99.8% absolute), acetone (Act, 99.5% absolute) and methanol (MeOH, absolute) were purchased from Sigma-Aldrich, Escherichia coli ATCC 25922 strain (0.5 McFarland), Proteus mirabilis and Klebsiella pneumoniae bacteria were obtained from Erciyes University Hospitals Central Laboratory, and Escherichia coli antibody ab25823 was purchased from Abcam Cambridge, UK. AIM-4300 Antenna Analyzer (5 kHz to 300 MHz) device was obtained from Array Solutions Sunnyvale, United States. 8B soft Staedtler pencil, Flormar brand nail polish, Bacillus clausii (Enterogermina 5 mL oral, Sanofi) and Neodymium Rectangle Magnet were obtained from Amazon Corp (Istanbul, Türkiye). All chemicals were used without further purification.
2.2. Fabrication of Graphite Sensors
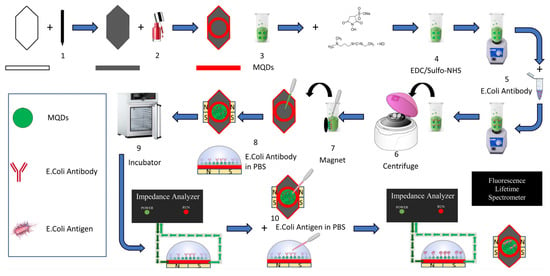
A 3.4 cm wide and 1.7 cm long sensor was created from standard A4 paper, weighing 80 g/m
2. A surface area of 4.25 cm
2 on the top of the sensor was painted with an 8B soft Staedtler pencil (
Figure 1. Step 1). Thus, a graphite-coated surface was obtained. The coating was continued by taking measurements with a multimeter from time to time until the targeted conductivity value of 3 mS was achieved. In order to have a circular working area of 1.3 cm
2 on this surface, approximately 1 cm
2 circular area outside this working area was covered with commercial nail polish to create a hydrophobic area (
Figure 1. Step 2). In addition, hydrophobic areas were created by completely covering the bottom and side of the sensor with nail polish. The fact that these individually produced sensors have the same conductivity value has been ensured by taking measurements with a multimeter unit.
2.3. Synthesis of Antibody-Modified CdSe/ZnS@Fe2O3 NPs
Synthesis of green-emitting QDs: For the synthesis of CdSe/ZnS quantum dots core/shellQDs, CdO (0.3 mmol) and zinc acetate (ZnAc, 4 mmol) were reacted with oleic acid (5 mL) in a three-neck flask. Evacuation is done until the vacuum level reaches 10
−3 torr at 100 °C. 15 mL of 1-ODE is added to the solution temperature before it exceeds 100 °C. When the mixture is at 100 °C, the evacuation process is continued and 10
−3 Torr vacuum level is attained. When the system reaches the desired vacuum pressure, the vacuum valve is closed and the N
2 gas flow valve is opened. The solution temperature is slowly adjusted to 300 °C. Se (0.3 mmol), S (3 mmol), 2 mL TOP solution is prepared in a glove box and the mixture is mixed to a clear solution at 65 °C and 750 rpm stirring speed. This prepared solution is taken from the glove box and injected into the solution that has reached 300 °C, and the mixture is cooled immediately after 10 min of reaction time. After green CdSe/ZnS QD synthesis, washing is done (with hexane, acetone and methanol) to separate them from the excess ligands in the medium [
33].
Synthesis of Yolk Iron Oxide Shell (Fe
2O
3) around the CdSe@ZnS Core: Vacuum-dried CdSe@ZnS nanoparticle sample (0.1491 g) was dispersed in a mixture of 20 mL ODE and OAm (the volume ratio of OAm/Fe(CO)
5; 1.43) with sonication. The mixture is evacuated in a basket-type heater at 120 °C until the vacuum level reaches 10
−3 Torr. When the vacuum value reaches the desired value, the vacuum valve is closed, the N
2 gas is opened and the temperature is adjusted to 60 °C. When the temperature reaches 60 °C, 2 mL of Fe(CO)
5 (Fe(CO)
5/CdSe@ZnS by weight ratio; 0.7) solution is injected into the mixture. After the injection, the basket-type heater is withdrawn and the reaction is continued with the sonicator. The sonochemical reaction is kept for 30 min to allow ligand exchange to occur. After 30 min, a metallic Fe shell was formed around the CdSe@ZnS core. After the sonication process was completed, the solution temperature was adjusted to 100 °C in the basket heater, and the solution consisting of oxidizing agent TMNO (0.012 g) and ODE (5 mL) was added, and at this point, controlled oxidation of iron was achieved with the TMNO/ODE mixture. This resulted in the formation of a yellow Fe
2O
3 iron oxide shell enclosing CdSe@ZnS. Superior magnetic properties emerged after oxidation. Washing was performed to remove ligands with the formation of CdSe/ZnS@Fe
2O
3 [
34]. Synthesis of MQDs is illustrated in
Figure 1. Step 3.
The formed amine:(CdSe/ZnS@Fe
2O
3); 15 mg of Sulfo-NHS were dissolved in pyridine (5 mL). EDC (15 mg) in 0.25 mL of chloroform was added to the above solution. 40 mg of CdSe/ZnS@Fe
2O
3 NPs was added, and then the mixture was shaken for 2 h. The formed amine:(CdSe/ZnS@Fe
2O
3) NPs were precipitated by the addition of hexane, separated by an external magnet, and washed with ethanol and H
2O [
35]. In this way, the sensor surface was functionalized as amine reactive.
Synthesis of antibody-(CdSe/ZnS@Fe
2O
3) NPs; 5 mg amine group modified magnetic quantum data were weighed and 180 µL pH (7.4) sodium phosphate buffer was added and sonicated to disperse the particles. In order to provide antibody modification, 20 µL amin active of
E. coli antibody with 10
15 concentration was added to this prepared solution. This mixture was sonicated for 30 min and then the reaction was completed in an orbital mixer for 45 min. Bacteria were made reactive with MQDs, EDC/Sulfo-NHS and antibodies as illustrated in
Figure 1 Steps 4 and 5. The mixture is centrifuged for about 1–2 min until it settles (
Figure 1. Step 6). The amine active antibodies were linked via a stable amide bond. At the end of the procedure, washing was done 3 times with a phosphate buffer to remove unbound antibodies.
2.4. Preparation of the Sensor Surface
The prepared antibody-(CdSe/ZnS@Fe
2O
3) NPs were held by an external magnet, 200 µL were dropped onto the detection surface of graphite-coated (
Figure 1. Steps 7 and 8) and polished paper-based biosensors and left to dry for 15 min at 40 °C in the incubator device (
Figure 1. Step 9). After the drying process, the sensors are ready for analysis with impedance and quantum efficiency.
2.5. Characterization Techniques for CdSe/ZnS@Fe2O3 NPs
Transmission electron microscopy (TecnaiG2-F30 TEM, FEI, Hillsboro, OR, USA) was utilized to analyze the morphology of yolk-shell CdSe/ZnS@Fe2O3 nanoparticles. A 2 μL droplet of the CdSe/ZnS@Fe2O3 solution, dissolved in hexane, was placed onto a copper grid and subsequently air-dried. The TEM employed a field emission electron source operating at 200 keV for imaging. Energy-dispersive X-ray (EDX) analysis was carried out on a Zeiss Gemini 300 model device (Oberkochen, Germany). Infrared (IR) spectra were collected on Thermo Scientific Nicolet 6700 FTIR spectrophotometer (Waltham, MA, USA).
2.6. Detection of E. coli Bacteria by Prepared Sensors
A magnet was placed under the modified MNPs (bare Fe
2O
3 as magnetic nanoparticle) and MQDs (magnetic Fe
2O
3 coated CdSe/ZnS) on the surface of the prepared multifunctional sensors to better contact the Carbon atoms on the graphite-covered surface by utilizing magnetism, and then they were connected to the probes of the electrochemical impedance spectroscopy device (AIM4300, Array Solutions, Sunnyvale, TX, USA) for impedance measurement. 150 µL of phosphate buffer was added on it and the first measurement was taken only when the antibody was bound. Then the buffer solution was drawn again and
E. coli bacteria (concentration of 10
8, 10
7, 10
6, 10
5, 10
4, 10
3, 10
2 CFU/mL was added in 150 µL phosphate buffer (
Figure 1. Step 10). Impedance measurements were taken at different times (t = 0, 10, 20, 30 min). After 30 min, the sensor surface was washed with phosphate buffer and unbound bacteria were removed from the sensor. After the washing process, impedance measurement was taken again when 150 µL of phosphate buffer solution on the sensor surface and the data were recorded.
In order to measure the bacterial specificity of the prepared device, in the above experimental procedure, measurements were made with E. coli, Proteus mirabilis, Klebsiella pneumoniae and Alkalihalobacillus clausii (Bacillus clausii) bacteria at a concentration of 106 CFU/mL in 150 µL.
In addition, test and control experiments with 10 repetitions each were performed to test the stability of the system. For this, the first solution containing E. coli, Proteus mirabilis, Klebsiella pneumoniae and Alkalihalobacillus clausii bacteria at a concentration of 106 CFU/mL in 150 µL, and the second solution containing Proteus mirabilis, Klebsiella pneumoniae and Alkalihalobacillus clausii bacteria at a concentration of 106 CFU/mL in 150 µL were prepared. And experiments were conducted as test and control respectively. In our research, all experiments were carried out in at least 3 repetitions.
Time-correlated single photon counting is a powerful technique to monitor the excitonic interactions on the emissive MQDs. We utilize this tool to simultaneously monitor the effect of the interaction between the bacteria and MQDs. After the process was finished, the changes in the irradiation kinetics of the bacteria-bound and non-bacteria-bound sensors were analyzed in a real-time device for each sample using PicoQuant FluoTime 200 (Berlin, Germany).
The raw data from all stages of the experiments were taken one by one and transferred to the data processing software, these data were manipulated with the nonlinear curve fit (parabole) process, as much as 1000 data, and fitting was applied. For the data that could not reach 1000 data, extrapolation was performed. Rs calculation was made by considering the points where the data intersects the x-axis.
2.7. QCM Characterization
To clean the gold surface of a 5 MHz AT-cut quartz crystal (Biolin Scientific–QSX 301, Gothenburg, Sweden), it was placed in a 10-fold de-ionized water (DIW) (Erciyes University Environmental Engineering, Kayseri, Türkiye) diluted solution of piranha (1.5 mL sulfuric acid (1120801000, Merck, Istanbul, Türkiye), 0.5 mL hydrogen peroxide (Merck) for at least 2 min. Then the sensor was washed sequentially with ethyl alcohol (Merck, 99.9%) and distilled water. The cleaned gold surface was exposed to a solution of 2.5 mM cysteamine (Merck) in de-ionized water for 12 h. Frequency shifts in the QCM crystal were monitored with an impedance analyzer (Array Solutions, AIM-4300, Sunnyvale, TX, USA). Amine-reactivated MQDs at a concentration of 5 mg/mL in 1× Phosphate Buffered Saline (PBS, 18912014, Thermo Fisher, Istanbul, Türkiye) solution was applied to the QCM sensor for 30 min by means of a peristaltic pump (Longer Pump, BT100-2J, Istanbul, Türkiye).
Detection System
After each measurement step, PBS washing was performed with a peristaltic pump for 5 min. Anti-E. coli antibody at a concentration of 1013 Ab/mL in PBS was applied to the sensor surface for 30 min. After the surface was blocked for non-specific binding by applying 1% BSA solution in PBS to the surface, the stability of the sensor in the negative control was followed for 30 min. In the last step, detection was completed by applying E. coli bacteria with a concentration of 107 CFU/mL in 1% BSA + PBS to the sensor surface.
3. Results
The FTIR spectra of the synthesized CdSe/ZnS@Fe
2O
3 nanocomposite and the bare CdSe/ZnS are shown in
Figure 2.
The characteristic peaks of CdSe/ZnS are around 3004 and 2900 cm−1 corresponding to the stretching vibrations of O-H and C-O bands, respectively. Two sharp peaks around 1540 and 1454 cm−1 were observed due to the stress vibrations of the thiol coating.
Additionally, for the CdSe/ZnS@Fe
2O
3 nanocomposite, the peaks around 694 cm
−1 in FTIR show the characteristic vibration peak of Fe-O and these peaks indicate the presence of maghemite [
36,
37].
Figure 3 reveals the elemental stoichiometry of the nanoparticles by Energy Dispersive X-ray analysis (EDX) (Zeiss, Gemini 300, (Oberkochen, Germany)) along with the transmission microscopy image. The observed peaks are consistent with the chemicals used. Since iron is in the form of a thin layer of chelate, it should appear in low amounts and the results are consistent with the amount of material used.
Table 1 shows the elemental analysis of the synthesized magnetic quantum dots.
The equivalent circuit model of the impedimetric biosensor is shown in Graphic Abstract in the impedance analysis measurements made at frequencies ranging from 5 kHz to 5 MHz [
38].
In the equivalent circuit model (
Figure 4a,b), R
m represents the impedance of the biosensor consisting of medium solution resistance, R
s electrode-solution interface resistance, C
s electrode-solution interface capacitance, C
p the parasitic capacitance and R
p parasitic resistance [
38].
The study conducted in
Figure 5 aims to compare MNP and MQD in terms of sensitivity based on the percentage change in the R
s value and to determine the time it takes for the sensor surface to reach saturation at a specific target concentration. Given at
Figure 5a,b, it is seen that R
s value also increases with bacteria bound as a result of antibody-antigen interaction. The R
s0 value in the related graphs shows the electrode-solution interface resistance in PBS when there are modified nanoparticles on the graphite sensor surface. R
s1, on the other hand, shows the electrode-solution interface resistance of the sensor in PBS after the washing process after the antibodies on the nanoparticle bind to the bacteria. R
s0 and R
s1 are used to show the percent change in relative electrode-solution interface resistance. It can be seen that the difference between R
s0 and R
s1 is less in
Figure 5a and more in
Figure 5b. This difference shows that MQD produces more sensitive results than MNP. Looking at
Figure 5c,d, it is seen that the R
s values, which are the basic criteria for bacteria detection, reach saturation in 30 min in both MNP and MQD sensors.
Experiments were started using 1 mg MNP in order to detect 10
7 CFU/mL
E. coli bacteria, as seen in
Figure S1. 18.57026 (±10.4499) %ΔR
s change was obtained. In the later part of the experiment, 5 mg MNP was tested and 24.94076 (±0.76582) %ΔR
s results were obtained. At this point, approximately 40% more change was achieved. Afterwards, 1 mg MQD was used. The change 37.72521 (±4.3315) %ΔR
s was obtained. 1 mg MQD produced approximately 50% more change than 5 mg MNP. Afterwards, 5 mg MQD was used and the change 52.434 (±6.7400) %ΔR
s was obtained. In this case, similar to the literature review, approximately 40% more change was obtained compared to 1 mg MQD and approximately 110% more change than 5 mg MNP [
39,
40]. With this comparison, it was decided to use 5 mg MQD in the further experiments.
To elucidate the role of quantum dots in enhancing sensor performance, we compared the electrochemical response of graphene + Fe
2O
3 + antibody electrodes with graphene + Fe
2O
3 + quantum dots (CdSe/ZnS) + antibody electrodes. Impedance spectroscopy revealed a ~52% relative change in ΔR for the quantum-dot-incorporated electrode compared to ~25% for the Fe
2O
3 –only electrode for 5 mg dry nanoparticle samples. This enhancement is attributed to the superior electrocatalytic activity and increased surface area provided by quantum dots, enabling more efficient charge transfer and greater antibody binding capacity. Furthermore, the quantum dots enable optical detection, offering dual detection capabilities that are absent in the Fe
2O
3 –only electrode. These results underscore the critical role of quantum dots in achieving higher sensitivity, specificity, and multimodal detection in the proposed hybrid biosensor (
Figure S1).
The functionality of the graphite sensor surface functionalization methodology used in the study has been confirmed by the QCM, which is valid in biosensor applications with the data made on the QCM and showing all the steps (
Figure 6a).
We demonstrated that the MQDs on the graphite sensor are functional by using a QCM sensor. By functionalizing the MQDs, which we immobilized on the QCM gold surface with a covalent bond, using the same cross-linking agents and receptor in our study, we demonstrated on the generally accepted [
41,
42,
43] mass sensitive QCM sensor that we can detect [
44,
45] bacteria with this functionalization method.
Using 5 mg magnetic quantum dots (MQDs), with E. coli bacteria at negative control (NC), 102, 103, 104, 105, 106, 107 and 108 CFU/mL concentrations, −0.2422 (±0.58), 0.0914 (±4.0532), 5.8769 (±7.2423), 15.0806 (±4.1235), 25.5584 (±1.1232), 37.3312 (±3.2752), 52.434 (±6.74) and 55.10 (±9.6033) ΔRs (%) changes were detected, respectively.
To determine the limit of detection and linear dynamic range of the biosensor, the curve whose equation is given between the concentrations of 10
3 CFU/mL and 10
8 CFU/mL, with a linear relationship observed in the graph in
Figure 6b, was fitted. The intersection point of the curve fitted and extended between negative control and 10
2 CFU/mL concentration and the curve reported above is seen. This point is the limit of detection point and its value is determined as 2.7
× 10
2 CFU/mL [
46].
In light of these results, it can be stated that the hybrid sensor platform is a highly sensitive and wide-ranging sensor, with a detection limit of 2.7 × 102 CFU/mL and a linear dynamic range of 103 CFU/mL to 108 CFU/mL, for detecting E. coli.
An
E. coli concentration of ≥10
5 CFU/mL in asymptomatic individuals, or
E. coli concentration of ≥10
2 CFU/mL in symptomatic patients, is typically considered as a UTI [
47].
The handmade paper-based graphite sensor, which has been variously modified to detect
E. coli bacteria, was specification tested with other bacteria that cause urinary infections in which
E. coli was detected. These bacteria are gram-negative like
E. coli,
Proteus mirabilis,
Klebsiella pneumoniae and also gram-positive
Alkalihalobacillus clausii (
Bacillus clausii). All bacteria were tested to a concentration of 10
6 CFU/mL. As seen in
Figure 6c, the system whose antibody of
E. coli bacteria is modified on the sensor surface does not respond to bacteria other than
E. coli. These values are respectively: for
E. coli (EC) 37.3312 (±3.2752) %ΔR
s, for
Bacillus clausii (BC) −0.2944 (±0.9840) %ΔR
s, for
Proteus mirabilis (PM) −1.6675 (±6.8444) %ΔR
s and for
Klebsiella pneumoniae (KP) 1.2737 (±1.0803) %ΔR
s. In this way, it shows that it is quite specific [
48].
As seen in
Figure 6d, 10 consecutive stability control experiments were performed with the system to detect
E. coli bacteria in the control medium containing
Proteus mirabilis,
Klebsiella pneumoniae and
Alkalihalobacillus clausii bacteria at a concentration of 10
6 CFU/mL in 150 µL, except for
E. coli bacteria. As a result of these experiments, a change of −0.2422 (±0.58) %ΔR
s was obtained.
Then as seen in
Figure 6c, 10 successive stability test experiments were performed with the system to detect
E. coli bacteria in the test medium containing
E. coli bacteria,
Proteus mirabilis,
Klebsiella pneumoniae and
Alkalihalobacillus clausii bacteria at a concentration of 10
6 CFU/mL in 150 µL. As a result of these experiments, a change of 27.4529 (±0.9823) %ΔR
s was obtained.
Looking at the dose response graph in
Figure 6b, it is seen that the change at 10
6 CFU/mL concentration is 37.3312 (±3.2752) %ΔR
s. But in the stability test, the change was 27.4529 (±0.9823) %ΔR
s. In other words, there is a decrease in change of approximately 27%. The reason for this was the presence of bacteria with similar characteristics in the detection environment and the interference of these bacteria.
The repeatability of each experimental set was calculated by the following equation:
σ represents the standard deviation of the concentrations measured in each experimental set and μ represents the mean value of the concentrations measured in each experimental set.
In
Figure 6d, black dots are control experiments and red dots are test experiments. As a result of the stability repeatability study, the relative standard deviation (RSD) (%) value of the sensor was found to be 3.5781, according to the formula mentioned above.
To inaugurate a novel path for bacterial detection and ensure the tangible perception of bacteria on the quantum dot’s surface, we employed time-correlated single photon counting measurements on magnetic quantum dot specimens. By modulating the bacterial concentration on the MQD, facilitated by the excitonic interactions between MQDs and bacteria, we methodically discerned accelerated emission decays in the samples. This acceleration signifies the extent of interaction, even discernible at exceedingly low bacterial concentrations.
Figure 6e presents the time-correlated single photon count decays of the MQD complex. Here, the same hierarchical procedure was utilized with the impedance measurements.
The bare lifetime of the quantum dots, before any bound E. coli on the surface have been measured as 8.59 ns. The lifetimes of the magnetic quantum dots were extracted as 3.37, 3.72, 3.92 and 4.66 ns, respectively, at 107, 106, 105 and 104 CFU/mL E. coli concentrations dropped on the sensor surface. As the concentration of the E. coli increased steadily from 104 to 107 CFU/mL, the average lifetime of the quantum dots decreased down to 3.37 ns demonstrating over 60% lifetime quenching.
With standard A4 paper, 8B pencil and nail polish, a biosensor that is very cost-effective, simple, fast, portable, sensitive, specific, stable and accurate measurements were obtained. CdSe/ZnS quantum dots with Fe
2O
3 magnetic shell, surface modified with amine groups, antibodies recognizing
E. coli bacteria attached to the detection point on the surface of graphite-coated paper biosensors were immobilized with the help of a magnet, and both an electrochemical and optical sensor were obtained. In this way, all the advantages desired to be obtained from an electrochemical biosensor and an optical biosensor were combined [
49,
50,
51].
While no additional external cross-check experiments were conducted, the developed hybrid biosensor was validated through its dual-mode detection approach (electrochemical impedance and optical fluorescence lifetime), specificity testing against non-E. coli bacteria, and repeated stability analyses. The consistency of results across these independent tests provides robust confirmation of the method’s accuracy. Furthermore, the sensor’s performance metrics, such as sensitivity, limit of detection, and specificity, are in agreement with previously reported studies, further corroborating the validity of the results. These validations demonstrate that the hybrid biosensor is a reliable tool for accurate E. coli detection.
4. Discussion
With the biosensor, whose cost is very low and sensor fabrication is very easy and fast, both electrochemical and optical measurements can be achieved at the same time. This is a measure to increase sensitivity and accuracy. Apart from this, using the nanoparticles with a diameter of around 13 nm, have a reasonably high active area, gives faster results in interaction with bacteria, and creates a sensor that can detect even small concentrations. The fabricated sensor and MQDs and their surface modifications were confirmed by electrochemical impedance spectroscopy (EIS) and fluorescence time-correlated single photon counting method (TCSPC) Through this approach, the sensor production methods are very simple, the sensor is produced in 10 min and the functionalized magnetic quantum dots to detect
E. coli can be made ready on the sensor surface within 90 min. Furthermore,
E. coli detection allows on-site measurements with electrochemical and optical detection systems on the sensor surface within 30 min. Different studies related to this subject have been investigated and our comparisons are given in
Table 2.
The UTI threshold for asymptomatic individuals is 10
5 CFU/mL and for symptomatic patients is 10
2 CFU/mL and when the table above is examined, our proposed hybrid biosensor is quite sensitive in detecting such low concentrations with a detection limit of 2.7 × 10
2 CFU/mL [
47,
59,
60]. Additionally, the sensitivity of our biosensor can be further increased with methods such as recombinant antibody technology [
61,
62], sandwich detection methodology [
63,
64], and accessory proteins such as protein A and protein G [
35,
65] that allow the correct orientation of the receptor.
In the studies summarized in the table, sensor preparation takes quite a long time. In our study, the sensor is made ready for detection in a short time of 90 min, and the detection system allows measurement by mutual verification with two different detection platforms. Another advantage of the dual measurement system in our study is that it makes an accurate and sensitive quantitative measurement with impedimetric measurement [
66,
67,
68]. In addition, MQDs that emit at different wavelengths can be specified for different strains and bacteria and a solution that would allow the detection of different strains and bacteria at the same time can be proposed.
The novelty of this study lies in the development of a hybrid biosensor that integrates paper-based electrochemical detection with optical sensing, utilizing magnetic quantum dots (MQDs) as the core sensing element. This dual-mode approach, which is not commonly reported in the literature, allows simultaneous detection using two complementary techniques, enhancing both sensitivity and accuracy. The MQDs, comprising CdSe/ZnS quantum dots with a Fe2O3 magnetic shell, provide a high surface-to-volume ratio for effective antibody immobilization and dual magnetic/optical properties. These features enable rapid sensor preparation (90 min), a low detection limit of 2.7 × 102 CFU/mL, and specificity in distinguishing E. coli from other bacterial species. Furthermore, the use of low-cost and easily accessible materials such as standard A4 paper, pencil graphite, and nail polish underscores the practicality of this sensor for point-of-care diagnostics. This work bridges the gap between high-performance detection systems and cost-effective, portable platforms, presenting a significant advancement in biosensing technologies.