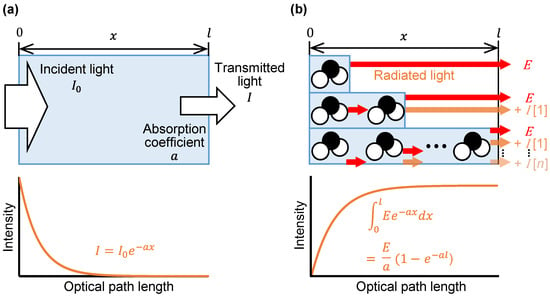
Next, we verified that not only the thickness of the substance, but also the absorption coefficient at each wavelength, is a variable in the emission integral effect. Because a large absorption coefficient means a large emission intensity, a large change in emission intensity can be expected at peak wavelengths with large absorption coefficients. Using mid-infrared passive spectroscopic imaging, we obtained the spectral radiance of polypropylene, which represents the intensity of emitted light at each wavelength. As shown in Figure 4a, the same polypropylene substances used in the experiment to prepare Figure 3 were measured from a distance of 600 mm. Figure 4b shows that the emission intensity at each wavelength increased as the substance thickness increased, and Figure 5 shows that the rate of this increase was greater at the peak wavelengths of 10.02 and 10.26 µm (where is large) than at the baseline wavelengths of 9.36 and 11.33 µm (where is small). Moreover, Table 1 shows the ratio of the mean values (values in Figure 4) to three times the standard deviation for the 30 replicates of this experiment. Approximately 99.7% of the data are expected to vary within ±6% of the mean, making this a precise and repeatable measurement. Therefore, consistent with Equation (1) and Figure 1b, the emission integral effect depends not only on the thickness of the substance, but also on the absorption coefficient at each wavelength. The spectral characteristics of the 3.00 mm thick polypropylene clearly show that a saturation-like behavior occurred, in which the intensities at the peak and baseline wavelengths were no longer significantly different when the intensity increased due to the emission integral effect became too great (Figure 4b). Therefore, when using mid-infrared passive spectroscopic imaging, it should be easier to identify the components of thinner materials and/or those with small absorption coefficients.
Source link
Daichi Anabuki www.mdpi.com