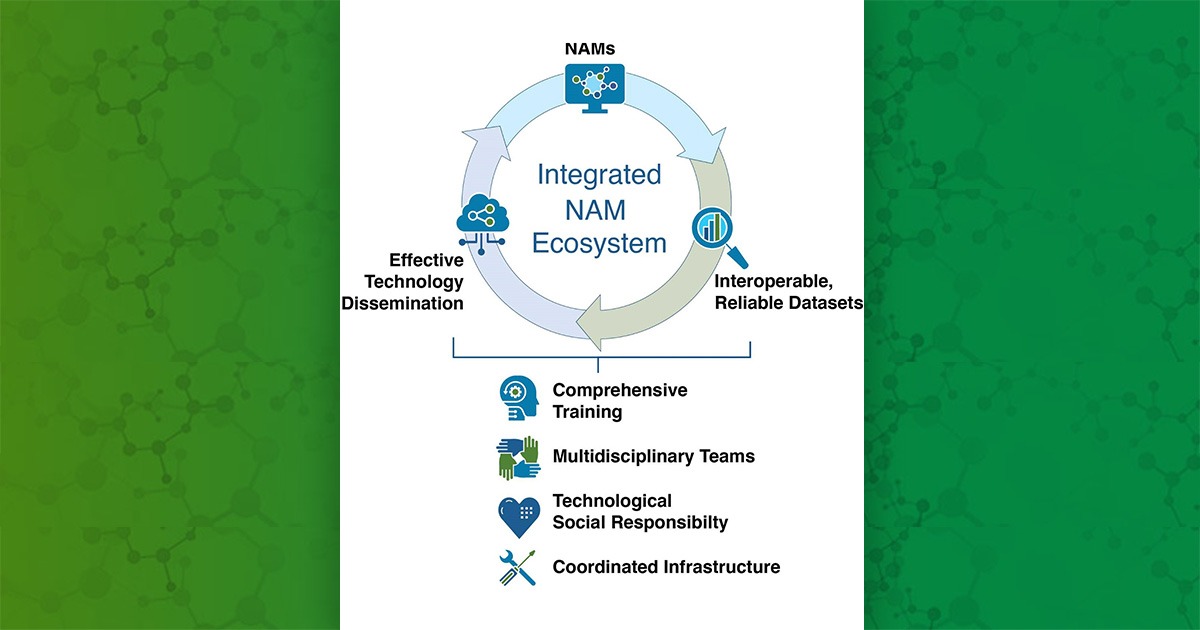
In a February 1 statement, National Institutes of Health (NIH) Director Monica Bertagnolli, M.D., accepted a working group’s recommendations on activities to advance development and use of non-animal methods in biomedical research. The statement highlighted the key role of an NIEHS office in advancing such methods, particularly with an eye toward validation and regulatory implementation.
Bertagnolli’s statement endorsed recommendations of the Working Group on Catalyzing the Development of Novel Alternative Methods to Advance Biomedical Research, which was established by the Advisory Committee to the NIH Director to identify high-priority areas for NIH investment in new approach methodologies (NAMs), including non-animal approaches for studying human biology.
“NIH accepts these recommendations and is committed to continuing its investment in building a robust suite of tools for researchers to study human biology and disease,” stated Bertagnolli. “These recommendations build on an existing foundation of NIH investment in NAMs projects.”
Bertagnolli noted the key role played by the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) to advance NAMs acceptance and use within the federal government. ICCVAM is a permanent committee of NIEHS under the National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM).
Working group included ICCVAM advisors
The role ICCVAM plays in advancing NAMs acceptance was of particular interest to two members of the working group, Antonio Baines, Ph.D., North Carolina Central University, and Szczepan Baran, V.M.D., VeriSIM Life, who also both serve on ICCVAM’s advisory committee.
Baines commented that the timing of the working group’s establishment reflects both a need for and a growing acceptance of broader use of NAMs.
“There are so many chemicals lacking adequate toxicity data, especially chemical mixtures,” he observed. “NAMs can be applied both to complement and to replace animal use in providing these data.”
Baran said that there are several areas in which ICCVAM and NICEATM can support the working group’s recommendations.
“Given their extensive engagement with diverse stakeholders, ICCVAM and NICEATM are ideally positioned to pinpoint and articulate current challenges while emphasizing the need and justification for these innovative tools,” he noted.
Both members felt that ICCVAM is well suited to support the working group’s recommendations on disseminating information about NAMs and encouraging agencies to craft policies that prioritize NAMs use.
Alignment with NICEATM and ICCVAM activities

Established in late 2022, the NIH working group held meetings throughout 2023, seeking public input via a published Request for Information in June and a public workshop in August. Presenters at the workshop included NICEATM Director Nicole Kleinstreuer, Ph.D., and ICCVAM members Elijah Petersen, Ph.D., National Institute of Standards and Technology, and Jessie Carder, U.S. Department of Agriculture.
Kleinstreuer noted how the working group’s recommendations align with ongoing NICEATM activities.
“NICEATM has long been exploring how combinations of NAMs can be used to improve on the ability of single NAMs to predict toxicity,” she commented, noting specific NICEATM efforts in the areas of skin sensitization and eye irritation. “We’re also playing a leading role in providing high-quality datasets and computational workflows through our Integrated Chemical Environment.”
Petersen and Carder both felt that ICCVAM’s activities in evaluating NAMs for specific regulatory uses are aligned with the working group’s recommendation on supporting a NAMs infrastructure.
“That is a clear present and ongoing role for ICCVAM, and there’s great potential for collaboration and productivity in this space,” commented Petersen.
The ICCVAM members also felt that their committee is in a unique position to facilitate collaborations and training on appropriate use of NAMs.
“We need more trust in NAMs, which I think will partly come from better transparency regarding the intended use and purpose of NAMs, and, more importantly, the limitations,” observed Carder.
(Catherine Sprankle is a communications specialist for Inotiv, the contractor supporting NICEATM.)
Source link
factor.niehs.nih.gov