2.2. Discusion
Horses share similarities with humans in the natural wound-healing process. In addition to both species developing different types of chronic wounds, horses serve as a relevant physiological model for studying wound repair mechanisms and evaluating potential therapies applicable in human clinical practice [2]. The skin architecture of humans and horses exhibits similarities, with both species having two primary layers that protect internal organs from mechanical damage and pathogen invasion. In humans, the epidermis comprises five layers of stratified epithelial cells, while in horses, it consists of stratified squamous cells. Both species have thick dermis layers, although humans have sparse hair follicles, while horses possess densely distributed follicles [26,27]. Studies on wound healing in horses, such as this one, may contribute as models for treating human skin lesions.
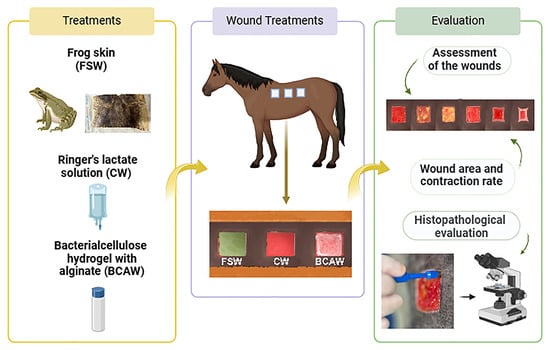
This study used sedation and local infiltrative anesthesia to ensure the animals’ comfort during surgical skin wound procedures, consistent with other experimental studies [28,29]. Additionally, non-steroidal anti-inflammatory drugs (NSAIDs) were administered during the first three days to effectively manage pain and edema, with only mild discomfort observed in the horses during dressing application in the initial post-surgical days. NSAIDs have similarly been employed in other studies on wound healing in horses [28,30,31], in which mild discomfort was also reported during dressing changes shortly after wound creation [32].
The design and size of the surgically-produced skin wounds, created with a template, were suitable for assessing treatments over an adequate period, as previously demonstrated [28,33]. The wound shape did not impact healing time in the horses [34], and using one side of the back for clinical evaluation and the opposite side for biopsy sampling prevented bias and interference in measuring wound area and contraction [29,30]. A clinical evaluation showed an initial inflammatory phase in all animals and groups following surgery, marked by hemorrhage, edema, hyperemia, and clot formation that persisted until D3 and was no longer evident by D7 [32].
In this study, exudate decreased as granulation tissue began filling the wounds but remained until closure. In CWs, exudate was noted until granulation tissue filled the wounds, after which they stayed dry. By D7, all animals had some granulation tissue, though not covering the entire wound area, similar to findings in other studies [28,35]. FSW exhibited three animals with prominent granulation between D14 and D21, and all groups on D28, but still with incomplete wound contraction.
Epithelialization of skin wounds began around D14 in most animals, becoming more pronounced in BCAWs on D21 and CWs on D28. Although other studies have shown a pro-healing effect, with good epithelial growth in frog skin-treated skin wounds in humans [23] and faster epithelialization in bacterial cellulose hydrogel-treated wounds in rats [36], this effect was not observed in our study. For both treatments (BCAW and FSW), no significant differences in wound area or contraction rate were observed compared to the control wounds by the end of the experiment (D28), consistent with findings in rat studies using bacterial cellulose hydrogel for experimental wounds [36]. The effect of cellulose gel with alginate on wound skin healing in horses is still lacking in the literature.
The pro-healing effects of cellulose gel combined with alginate are primarily attributed to its ability to retain moisture, creating an environment conducive to epithelialization by promoting epithelial cell migration into the moist wound area [36,37]. This property offers a significant advantage over bacterial cellulose alone or occlusive dressings, which generally maintain a low-humidity wound bed [38]. Studies on bacterial cellulose (BC) films incorporated with alginate, gelatin, and glycerol have shown that formulations made exclusively of BC and alginate exhibit excellent mechanical properties, such as high resistance and tensile strength, along with optimal hydrophilicity and a microenvironment favorable for keratinocyte and fibroblast growth [39]. Furthermore, bacterial cellulose/alginate hydrogels have demonstrated clinical efficacy; for example, in treating a diabetic ulcer, twice-weekly applications over 30 days led to an 84% reduction in wound size compared to the initial measurement [40].
Frog skin dressings have also shown promising healing properties, attributed to their porosity, which facilitates oxygen flow while preventing liquid or exudate buildup in rabbits [22] and humans [23]. Despite these demonstrated pro-healing effects of BC and alginate-based dressings and frog skin in other species, this study did not observe such effects in horses.
Despite minor variations, histopathological evaluations showed no significant differences across groups in terms of inflammatory response or wound healing markers, such as fibroblast presence and neovascularization scores. Although not statistically significant, BCAW demonstrated a slight reduction in neutrophil and histiocyte scores during the early and middle stages of healing. The cellulose/alginate gel has been shown to reduce neutrophil and macrophage activation, modulate the inflammatory phase, and enhance the healing of skin burns in rats [6]. It has also been associated with improved local perfusion and tissue metabolism in lumbar wounds in horses [9]. In rats, the topical application of frog skin lipid extract accelerated wound healing, likely due to its ability to shorten the inflammatory phase [41]. However, this effect was not observed in our study, similar to findings in dog integumentary wound healing [42]. Consequently, the wound-healing benefits of frog skin and bacterial cellulose hydrogel with alginate were not confirmed for experimentally induced skin wounds in horses.
Source link
Rita C. Campebell www.mdpi.com