1. Introduction
Thyroid cancer is the most commonly diagnosed malignancy of the endocrine system, with an increasing global incidence over the past few decades [
1]. It accounts for approximately 2% of all cancers and is predominantly diagnosed in women, with a notable age-related distribution, often affecting individuals between the ages of 20 and 55 [
2]. The majority of thyroid cancers are differentiated, including papillary and follicular subtypes, which typically have a favorable prognosis. However, more aggressive forms, such as anaplastic and medullary thyroid cancers, are less common and are associated with poorer outcomes. Advances in early detection, imaging techniques, and improved surgical treatments contribute to an overall improvement in survival rates, yet regional and metastatic disease remains a significant concern [
3].
Studies have identified various clinical and pathological factors that affect the prognosis of thyroid cancer patients. The main prognostic factors include age at diagnosis, gender, tumor size, lymph node involvement, extrathyroidal spread, and distant metastasis [
4]. However, the most important prognostic factor is the tumor’s histological subtype. Papillary thyroid carcinoma (PTC) is generally associated with a better prognosis, while follicular, medullary, and anaplastic thyroid cancers (ATC) tend to have worse prognosis [
5]. Studies in thyroid cancer also highlight the role of genetic mutations, such as those in the BRAF and RAS genes, which are linked to more aggressive disease and poorer survival outcomes [
6]. Understanding prognostic factors is critical for tailoring individualized treatment plans and improving patient outcomes.
Today, the incidence of thyroid cancer has increased significantly, especially in small and localized PTCs, probably due to the increased use of neck ultrasonography and fine needle aspiration (FNA) [
7]. This increased detection rate has led to earlier diagnosis, but it has also raised concerns about overtreatment. Economic, social, and demographic changes occurring in societies may lead to lifestyle changes in areas such as nutrition, smoking, alcohol use, and sports [
8]. These changes necessitate a re-evaluation of prognostic factors and survival outcomes using large population-based databases. In this study, we evaluated the changes in clinicopathological and survival characteristics of thyroid cancer patients in the SEER database between 2001–2010 and 2011–2020.
2. Materials and Methods
2.1. Patients and Data Selection
This study was designed as a retrospective cohort study. The patients included in the study were obtained from the SEER Program of the National Cancer Institute (NCI), and the patient data were anonymized. The study was conducted in accordance with the declaration of Helsinki and good clinical practice guidelines. We obtained data for this study from the US NCI’s SEER-12 registry [November 2023 (1992–2021)]. SEER*Stat program version 8.4.4 was used to extract data. The patient population to be included in the study was determined by selecting Site recode ICD-O-3/WHO 2008 = ‘’thyroid” and Behavior code ICD-O-3 = ‘’malignant’‘ from the SEER*Stat program. Patients to be included in the study were restricted as Age recode with < 1-year olds = “20–24, 25–29, …, 80–84 and 85 years +’’ and Year of diagnosis= “2001, 2002, 2003, …, 2019, and 2020”. From the SEER*Stat program, patients’ Sex, Race recode, Origin recode, AYA site recode 2020 Revision, Histologic Type ICD-O-3, Combined Summary Stage (2004+), Summary stage 2000 (1998–2017), RX Summ–Scope Reg LN Sur (2003+), Radiation recode, Chemotherapy recode (yes, no/unk), and SEER cause-specific death classification data was saved.
The patient group was divided into two cohorts. Patients registered between 2001–2010 were considered Cohort 1, and patients registered between 2011–2020 were assigned to Cohort 2. Patients’ ages were divided into 20–39, 40–59, and 60+. Patients registered according to AYA site recode 2020 revision were recorded as “Other” except for papillary, papillary with follicular variant (FV), follicular, hurthle cell, and medullary. Surgery status, according to RX Summ-Surg Prim Site (1998+), and lymph node sampling status, according to RX Summ-Scope Reg LN Sur (2003+), were divided into three groups as “Yes, No and Unknown”. Radiation recode is divided into four groups as: “Radioisotopes, Radiotherapy (Implant and/or external), Radioisotopes+Radiotherapy, and None/Unknown”. While calculating the overall survival (OS) of the patients, “Survival months” were taken as the duration, and analysis was performed with patients coded “Dead (attributable to this cancer dx)” according to SEER cause-specific death classification. In addition, univariate and multivariate analyses were performed to determine the factors affecting OS.
2.2. Statistical Analysis
The SPSS 25.0 software (IBM, Armonk, NY, USA) and MedCalc statistical software version 23.0.9 (Ostend, Belgium) were used to perform the study statistics. The characteristics of patients in Cohorts 1 and 2 were compared using the Chi-square test. We used the Kaplan–Meier technique to analyze survival. Cox regression test was performed for univariate and multivariate analysis to evaluate the factors affecting survival in patients with thyroid cancer. Hazard ratios (HRs) and their 95% confidence intervals (CI) were estimated. Statistically significant variables from the univariate model have been included in the multivariate model. Chemotherapy, radiotherapy, radioisotope therapy, and surgical treatments were not included in multivariate analysis because they were correlated with tumor stage at diagnosis in patients with thyroid cancer. Statistical significance was accepted as a p-value < 0.05.
4. Discussion
In this study, we compared the clinical features and prognoses of patients with thyroid cancer registered between 2001–2010 and 2011–2020 in the SEER database. Over the past several decades, advancements in early detection, treatment strategies, and molecular profiling have significantly influenced various cancers’ clinical presentation and prognosis. In particular, improvements in screening methods (such as mammography for breast cancer and colonoscopy for colorectal cancer) have contributed to earlier detection and, consequently, improved survival rates. In our study, comparing Cohort 1 and Cohort 2, we found that the proportion of patients aged 60+ and male patients tended to increase in recent years. Few studies in the literature have examined trends in thyroid cancer demographics with different findings regarding age and gender distributions. A study analyzing US data from 1976 to 2005 also observed that PTC incidence rates increased faster in women than in men, with the gender gap widening over time [
9]. In particular, the female-to-male incidence ratio decreases with age, from over five in individuals aged 20–24 to approximately one in individuals aged 80 and over, showing a convergence in incidence rates between the sexes in older age groups. Consistent with our findings, a study conducted in the Balearic Islands between 2000 and 2020 reported that the mean age at diagnosis increased from 47.3 years in the previous decade to 52.1 years in the following decade [
10]. These changes may reflect aging populations and improved diagnostic practices that detect thyroid cancer in older adults. However, gender trends remained consistent in this study, with proportionally similar thyroid cancer incidence in women compared to men throughout the study period.
One of the significant results we found in our study was the changes in the disease stages at diagnosis in the patients. Although diagnostic possibilities have increased over the years, we have shown that, contrary to expectations, there has been an increase in the proportion of patients in the regional stage and a decrease in the proportion of patients diagnosed with localized disease. A study conducted in Switzerland between 1998 and 2012 noted a significant increase in early-stage thyroid cancer diagnoses without an increase in advanced-stage cases, reinforcing the idea that less aggressive cancers are being overdiagnosed due to improved detection technologies and diagnostic practices [
11]. This pattern contrasts with the decrease in rates of early-stage disease observed in our study and likely reflects differences in health systems or population characteristics. Another analysis noted that shifts in staging criteria, such as the adoption of the eighth edition of the TNM staging system, led to systematic downstaging of many cases of differentiated thyroid cancer [
12]. This can be useful for interpreting trends over time, as changes in staging systems can affect the proportion of cases categorized as localized or regional. Direct comparisons of stage distributions are further complicated by the focus of modern staging systems on predicting mortality rather than recurrence.
We found a 5.3% increase in the frequency of PTC in the histopathological subgroup assessment in Cohort 2. Similarly, many studies have reported an increase in the frequency of PTC due to advances in diagnostic techniques such as high-resolution ultrasound and FNA biopsy. Similarly, this increase has been observed in various populations. For example, studies have shown that the incidence of PTC has more than doubled in certain regions, including South Korea, in recent decades, and this has been attributed primarily to increased screening efforts [
13]. This trend has also been confirmed in other countries, with studies highlighting a significant rise in PTC diagnoses due to the more widespread use of imaging and pathological examinations [
14]. Additionally, molecular alterations, such as those in the TERT promoter, have been shown to correlate with more aggressive PTC subtypes, further complicating the prognosis and treatment strategies [
15].
In the survival analysis of patients with thyroid cancer, we determined that age, gender, origin, stage at diagnosis, and histopathological subtypes significantly affected survival statistically. The best prognostic group in histopathology was papillary + FV, while the worst was the other group, which mainly included ATC and sarcomas. Few studies in the literature have also examined prognostic factors affecting survival in thyroid cancer patients. A population-based study found that tumor differentiation status, age, and stage at diagnosis were strong predictors of survival, with increasing age being associated with lower relative survival for each histological type [
16]. Similarly, another study reported that age greater than 60 years is associated with a worse prognosis in patients with PTC [
17]. However, while race is a significant determinant in some studies, it appears to interact with other factors like age and stage rather than acting as an independent prognostic factor [
18]. In the literature, there are studies on factors affecting overall survival in patients with thyroid cancer, as well as different studies examining factors affecting recurrence. In a recently published study evaluating factors predicting recurrence in patients with early-stage thyroid cancer, the presence of extrathyroid tumor extension and neck lymph node metastasis in patients with PTC were found to be statistically significant prognostic factors [
19].
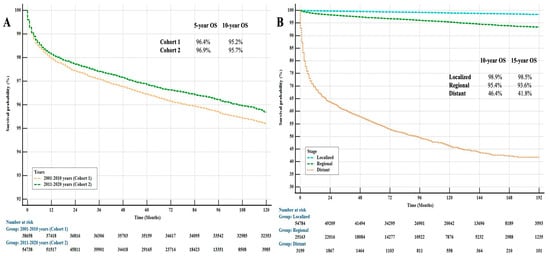
When comparing the cohorts in terms of prognosis, we found that, although the frequency of patients with regional-stage disease was increased in our study, there was a trend towards improved prognosis in patients with thyroid cancer in Cohort 2. There was an 8% survival improvement in patients in Cohort 2 compared with Cohort 1. This situation is thought to be possible due to the new drugs that have been used in cancer treatment in recent years. Several studies have examined the impact of new therapies on the prognosis of thyroid cancer, finding similar trends of survival improvement linked to novel therapies. In a recent study, it was found that combining targeted therapies and immunotherapy significantly improved overall survival in patients with ATC [
20]. Additionally, studies on ATC, which is known to be aggressive, demonstrate significant survival improvements over the past two decades with personalized treatment regimens that include targeted therapies such as BRAF mutation inhibitors [
21]. Similarly, in a study focused on differentiated thyroid cancer with lung metastases, it was indicated that radioiodine therapy (RAI) had a positive effect on survival, particularly in younger patients and those with fewer metastases [
22].
This study has some limitations as it is a retrospective cohort study. It is difficult to demonstrate causal relationships in retrospective studies as they cannot account for all possible confounding variables since the results have already been reported. In addition, different studies or institutions may have different standards for the quality and consistency of data collection, which may have led to a heterogeneous collection of information. Retrospective studies using historical data may lead to a sample that is less representative of the current population, which may limit the generalizability of the results. In addition, The NCI SEER Dataset is a very comprehensive database containing data on cancer patients. This database was created with data from many different cancer centers [
23]. The differences in diagnosis, treatment, and imaging methods among cancer centers are a limitation in obtaining standardized data. Also, there are deficiencies regarding patient treatment data in the NCI SEER Dataset [
24]. This situation makes it difficult to perform more in-depth statistical analyses.
5. Conclusions
In this large-scale study of twenty years of data from patients with thyroid cancer, we investigated the main factors affecting survival outcomes and prognosis. In the study, we revealed significant differences between the two cohorts; we found an increase in the proportion of older patients, males, and PTC in Cohort 2. Though an increasing trend in survival rates was observed in Cohort 2, the results were not statistically significant. We found that age, sex, gender, origin, stage at diagnosis, and pathological subtype, including papillary + FV subtype, were critical prognostic indicators. The strengths of this study were its large sample size and the comprehensive examination of survival data across multiple variables, which increased the reliability and generalizability of the study. Identifying age, gender, and cancer subtype as essential factors for prognosis may facilitate clinical decision-making and guide more personalized treatment strategies for thyroid cancer patients. In addition, by demonstrating changes in patient characteristics and survival over time, this study sets a valuable precedent for future studies to improve patient outcomes and understand the evolving nature of thyroid cancer.