The present study investigated possible electrophysiological interactions of sacubitril with the two QT interval shortening agents pinacidil and ouabain. The main results are as follows:
4.1. Combination of Pinacidil and Sacubitril
In the present study, pinacidil mimicked the electrophysiologic effects observed in short QT syndrome by activating IK,ATP and thereby reducing cardiac repolarization duration, subsequently facilitating the occurrence of arrhythmias and leading to an increased ventricular vulnerability. Another proarrhythmic mechanism of pinacidil is explained by the inhomogeneous distribution of IK,ATP, which is more expressed in the epicardium, thereby creating an inhomogeneous spatial repolarization and a substrate for phase 2 re-entry [23]. These present results are in line with previous studies in which pinacidil was used for the simulation of congenital short QT syndrome [10].
Additional sacubitril treatment had only minor effects on cardiac repolarization duration (reduction in APD90 by 3%; no change of QT interval). Of note, the reduction in effective refractory periods is often regarded as the main arrhythmic mechanism in shortened repolarization.
To better contextualize the effects induced by sacubitril, it is essential to consider its sole impact on cardiac electrophysiology. We have previously described the electrophysiologic effects of sacubitril in drug-naïve hearts: In this study [2], administration of 5 µM sacubitril had much more pronounced effects on cardiac repolarization in drug-naïve hearts (reduction in APD90 by 24% and of QT interval by 13%). In this prior study [2], 3 µM and 10 µM sacubitril also led to an abbreviation of APD90 (reduction by 18% (3 µM) and by 17% (10 µM)) and QT intervals (shortening by 10% (3 µM) and by 5% (10 µM)), though the effects induced by 3 and 10 µM were less pronounced than with 5 µM. Spatial dispersion of repolarization was reduced at each concentration, while the effective refractory periods were not significantly altered. No proarrhythmic effects were observed at any concentration.
In the same study [2], drug-induced long QT syndromes type 2 and 3 were simulated by administration of erythromycin or veratridine, respectively. In the long QT type 2 group, additional sacubitril treatment abbreviated cardiac repolarization duration and reduced spatial dispersion of depolarization. Thereby, sacubitril reduced the occurrence of early afterdepolarizations and torsade de pointes tachycardia. In contrast, further sacubitril treatment did not significantly alter repolarization duration and consequently did not exert antiarrhythmic properties in the long QT syndrome type 3 group.
To summarize, combined treatment with the repolarization shortening agents pinacidil and sacubitril has no significant additive impact on cardiac repolarization duration or arrhythmic risk. These findings are important since, as mentioned above, sacubitril alone has a substantial impact on cardiac electrophysiology. However, when administered in addition to pinacidil-pretreated hearts, sacubitril does not significantly alter repolarization. One possible explanation is that sacubitril may influence ion currents that have already been affected by the pinacidil pretreatment. To be more precise, sacubitril could open IK,ATP channels, which are already activated by pinacidil. However, a previous study demonstrated that sacubitril did not induce endothelial KATP, undermining this possible explanation [24]. In contrast, the combined administration of different QT-prolonging agents results in an additive lengthening of cardiac repolarization and ultimately in a relevant arrhythmic risk [25]. Other potential mechanisms underlying the sacubitril-induced shortening of repolarization include enhanced IKs or IKr, or reduced INa,L or ICa. Notably, these are the major currents that influence the proarrhythmic risk and are therefore considered in The Comprehensive in Vitro Proarrhythmia Assay (CiPA) initiative for assessing the safety of new drugs [26]. It is likely that sacubitril does not exert ion channel-specific effects, but instead has complex effects on cardiac ion channels. Since no experimental studies have been conducted to further elucidate this, these hypotheses remain speculative [2].
Notably, cardiac KATP channels are inhibited by physiological intracellular ATP levels, linking cellular metabolism with membrane potential [27]. As a result, ischemia activates IK,ATP and thereby abbreviates action potential duration and decreases ICa,L, thus protecting the cell from calcium overload and enhancing cell survival. It is important to note that the shortening of the action potential during acute ischemia primarily occurs early in the plateau phase [28,29]. These pathophysiological effects underscore the crucial role of IK,ATP activation in ischemic preconditioning [27]. However, the downside is that KATP activation, in addition to shortening the action potential, leads to the accumulation of extracellular K+ and a reduced conduction velocity, which can facilitate re-entrant ventricular tachycardias [27]. Another proarrhythmic mechanism is attributed to the inhomogeneous distribution of IK,ATP, which is more pronounced in the epicardium. This creates a heterogeneous repolarization, providing a substrate for phase 2 re-entry [23]. Due to these effects, pinacidil is commonly used to simulate electrophysiological conditions during ischemia [23]. In this light, sacubitril exerts a safe electrophysiologic profile in a model of acute ischemia, lacking proarrhythmic effects despite its repolarization-shortening properties.
4.2. Combination of Ouabain and Sacubitril
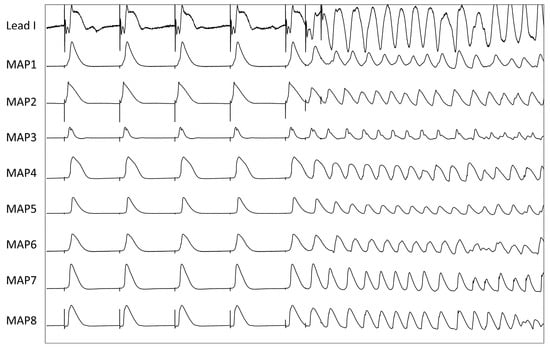
Treatment with ouabain also reduced cardiac repolarization duration and effective refractory periods. There are different potential mechanisms that might contribute to the action potential shortening effect of digitalis glycosides: (1) Digitalis glycosides inhibit the Na+-K+-ATPase, augmenting the intracellular sodium and calcium concentration and thereby increasing potassium permeability. This in turn increases the outward current during the plateau phase and abbreviates the action potential [12]. (2) Increased levels of cytosolic calcium accelerate calcium-dependent inactivation of the L-type calcium channel, reducing ICa,L and thereby shortening action potential duration [30].
No significant proarrhythmic effects were observed in the present study under ouabain infusion alone. Additional administration of sacubitril had a significant impact on cardiac repolarization as APD90 was abbreviated by 20%, QT interval by 13% and ERP by 18%. The underlying mechanism for these effects may involve an increase in IKr, IKs, or IK,ATP, or a decrease in INa,L or ICa. The shortening of cardiac repolarization duration, and particularly the refractory periods, can promote re-entry and potentially explain the increased occurrence of ventricular arrhythmias in the presence of sacubitril. The abbreviation of ventricular refractoriness facilitates re-entry by shortening the wavelength of the re-entry circuit [31].
However, it may be too simplistic to attribute the arrhythmic risk solely to the abbreviation of cardiac repolarization duration. There are other mechanisms that contribute to arrhythmias with digitalis glycosides such as an amplification of autonomic activity [32], which can be ignored in this study due to the experimental setup. In addition, digitalis glycosides can trigger delayed afterdepolarizations by spontaneous calcium release from the sarcoplasmatic reticulum due to calcium overload and CaMKII-mediated modifications of the ryanodine receptor [33] and thus promote polymorphic ventricular tachycardias [13,14]. The observations from this study are not the first to suggest an increased arrhythmic risk when combining digitalis glycosides with other drugs that influence cardiac electrophysiology. The PALLAS trial (Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy) [34] demonstrated that dronedarone increased the risk of death from cardiovascular causes in patients with permanent atrial fibrillation who were at risk for major vascular events. This adverse effect was at least in part driven by arrhythmic events under comedication with digoxin [35] and can be attributed to higher digoxin serum levels on dronedarone and a substantial abbreviation of cardiac repolarization [15].
Source link
Christian Ellermann www.mdpi.com