1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disease that is characterized by defects in motor activity, including resting tremor and muscular rigidity, as well as nonmotor symptoms, such as cognitive changes, dementia, and sleep disorders [
1]. In particular, the degeneration and loss of dopaminergic neurons from the substantia nigra region of the brain has been implicated in the presentation of motor symptoms in PD [
2,
3]. While both genetic and environmental factors have been implicated, the loss of neurons is often accompanied by the formation of misfolded toxic proteinaceous inclusions called Lewy bodies [
4]. The aggregation of these proteinaceous inclusions involves the interactions of several proteins, including synphilin-1, α-synuclein, parkin and ubiquitin C-terminal hydrolase L1 (UCH-L1) [
5,
6,
7]. Of these, one of the major proteins implicated in the progression of PD is alpha-synuclein (ASyn) [
8]. While not fully understood, the main function of ASyn has been linked to the control of neurotransmitter function and the neuromuscular system [
9], and its misfolding and loss of function results in deleterious effects. In addition, PD progression has also been linked to oxidative stress due to free radical damage [
10]. Approximately 5–10% of Parkinson’s cases are associated with mutations in PARK genes, which increase oxidative stress [
11]. Mutations in SNCA, a PARK gene that encodes alpha-synuclein, have also been implicated in the early onset of PD [
12].
Several reports have indicated the detection of copious amounts of ASyn oligomers and fibrils in Lewy bodies in the early stages of PD [
13,
14,
15,
16]. Neurons associated with oligomeric ASyn have been reported to have a higher level of oxidative stress. Furthermore, ASyn oligomers have been shown to induce ROS production and lower the levels of reduced glutathione [
17]. Additionally, ASyn oligomers produce superoxide radicals by binding to transition metal ions such as copper and iron [
18], and iron overload has also been implicated in oxidative stress, given its ability to modify ASyn upon binding. [
19,
20]. Furthermore, oxidative stress can also affect ASyn toxicity and mediate PD pathogenesis [
21,
22]. Thus, several studies are being conducted to elucidate the protein misfolding process of ASyn, and investigate ways to mitigate oxidative stress and misfolding. For example, to impede oxidative stress, curcumin, a natural antioxidant, has been recommended as a candidate drug for the potential treatment of PD, as it may replenish glutathione levels [
23,
24]. Several other natural antioxidants, such as lipoic acid, melatonin, carnitine, selenium, and natural polyphenols, as well as vitamins A, C, and E, have also been found to delay the progression of PD by reducing ROS levels [
25]. In another study, researchers have shown that antioxidant nanoparticles prepared by the self-assembly of ferulic acid and tannic acid components with adipic acid as a linker reduced alpha-synuclein aggregation and lowered pro-inflammatory cytokines [
26]. Although the administration of levodopa in conjunction with carbidopa or amantadine is one of the current mainstay treatments for PD [
27,
28,
29], several other approaches are also being investigated. Furthermore, hydrogen-inhalation therapy has been shown to decrease activated microglia and pro-inflammatory cytokines, particularly in the case of levodopa-induced dyskinesia [
30]. Monoamine oxidase type B inhibitors, such as rasagiline and selegiline, have been developed to enhance the use of dopamine by neurons [
31]. However, the continued administration of dopamine-related drugs has been linked to a relative loss of efficacy over time [
32].
More recently, peptide-based therapeutics have gained traction [
33,
34,
35,
36] due to their specificity and reduced toxicity. Current peptide-based approaches include utilizing brain-gut peptides that have been shown to have neuroprotective effects [
37], including the glucagon-like peptide-1 receptor agonist GLP-1. It has been shown to protect against neurotoxin damage to the dopaminergic system in mice [
38], restore levels of neurotransmitters depleted in PD, and alter cellular production and accumulation of amyloid beta (Aβ) deposits [
39,
40]. In another study, NPT-100-18a, a cyclic peptidomimetic compound derived from the peptide KKDQLGK, which interacts with membrane-bound Asyn, has been shown to disrupt and reduce the formation of oligomers in lipid membranes and reduce aggregation [
41]. Small molecule disaggregators of ASyn and prion-like protein aggregates such as Anle138b [3-(1,3-benzodioxol-5-yl)-5-(3-bromophenyl)-1H-pyrazole] have also been developed [
42,
43,
44].
Previous studies have also utilized computational methods such as molecular docking and molecular dynamics (MD) simulations to investigate the stability and binding interactions of various targets of PD. For example, the neuroprotective effects of the flavonoid karanjin were evaluated using molecular docking and molecular dynamics for five targets of Alzheimer’s disease (AD) and four targets of PD [
45]. Docking scores showed comparatively higher potency against AD and PD than standard drugs. Another study examined the effect of flavonoids, including morin, quercetin, and myricetin, on Asyn fibrils using MD simulations [
46]. They identified that the flavonoids destabilized the beta-sheet structure of Asyn fibrils and changed their morphology, with myricetin inducing the highest disruption, opening the potential for their use as a therapeutic to destabilize ASyn fibrils.
In this work, we have designed new peptides and utilized a biomimetic peptide-based computational approach to investigate their interactions with pathogenic fibrillar and filamentous segments of ASyn. To do so, several marine bioactive peptides were initially evaluated. The novel peptides were then designed using a peptide scrambling approach [
47], where antioxidant peptide motifs were conjugated with short sequences of fibril inhibitory motifs (FIMs). The selected antioxidant peptide segments were derived from natural sources such as mussels, ark shell scapharca subcrenata, or the C-terminal superoxide dismutase domain of Arthrospira Platensis [
48,
49,
50]. Each of the peptides has been shown to display antioxidant activity. To design the peptides, we created mutants of those peptide motifs to potentially further enhance their antioxidant activities. The FIMs were then connected at the N- or C-terminal or both ends of selected antioxidant motifs in order to develop peptides that may potentially mitigate ASyn fibrillation. In previous work, the FIMs (DPNGS and ELAQM) were shown to inhibit the fibrillation of insulin [
51]; however, these have never been tested in conjunction with other peptides against ASyn. In total, 14 peptide sequences known for their antioxidant activity were screened using the AnOxPePred web server (
https://services.healthtech.dtu.dk/services/AnOxPePred-1.0/ (accessed on 15 December 2023)) to predict their free radical scavenging activities. Those peptides were then mutated to design 40 new peptides to investigate if their antioxidant activity could be enhanced (
Supplementary Information Table S1). Based on those results, we created 20 new peptide sequences by attaching selected antioxidant motifs to FIMs. Upon the attachment of the FIMs, the new peptide sequences were again screened through AnOxPePred to ensure that the peptides demonstrated antioxidant properties (
Supplementary Information Table S2). Based on the most optimal results, 12 of those peptides were selected and investigated for their potential to bind to pathogenic fibrillar and the Lewy body-derived segment of filamentous ASyn and subjected to molecular docking studies and MD simulations to examine the binding interactions of those peptides with alpha-synuclein. We chose to examine both fibrillar and filamentous ASyn, as previous studies have demonstrated that different strains of ASyn possess different secondary structures, which lead to diverse levels of toxicity and propagation properties [
52] that may result from the exposure of different regions in their fibril structures [
53].
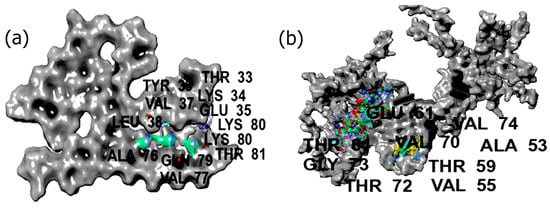
To validate the computational results, we examined five of the designed peptides and explored their binding interactions with ASyn fibrils through surface plasmon resonance (SPR) analysis. Furthermore, the impact on secondary structures of ASyn fibrils was examined through circular dichroism (CD) spectroscopy, where treatment with all peptides showed a reduction in the antiparallel beta-sheets over time, which was also corroborated through Thioflavin-T assay. Overall, this work establishes a basis for investigating the impact of short antioxidant peptides combined with FIMs and their influence on structurally different ASyn structures. It provides the groundwork for understanding the mechanism of binding interactions and the conformational changes involved in α-synucleinopathies for developing drug candidates for the potential treatment of PD.
4. Prediction of Pharmacokinetic Properties
The web server ADMETlab2.0 was used to predict the pharmacokinetic properties of all 12 of the designed peptides. The results obtained are shown in
Table 7. All peptides were accepted by the Pfizer rule (Lipinski’s rule of five). This rule aims to assess druglike behavior, including physiochemical properties, aqueous solubility, permeability, and oral bioavailability [
109,
110]. The predicted logP values represent the predicted partition coefficient for each of the peptides between the aqueous and lipophilic phases. In general, the logP values varied from −5.150 to 0.579, with ELAQMGPEGPMGLEDPNGS having the lowest score and PYYYWKELAQM having the highest score. This is likely due to the presence of several negatively charged residues in the case of ELAQMGPEGPMGLEDPNGS, with a pI of 2.87, while PYYYWKELAQM demonstrated a pI value of 6.85 (near neutral). The MDCK cell permeability values indicate that the peptides are generally expected to permeate the cellular membrane. The highest permeability was found for EQALMGFYGPTEDPNGS at 4.2 × 10
−6, and the lowest was found for ELAQMPYYYWKDPNGS at 9 × 10
−7. Furthermore, the peptides were not found to be hERG blockers, indicating that these will not cause cardiotoxicity related to hERG channel inhibition [
111]. The likelihood of the peptides acting as P-glycoprotein (Pgp) or multidrug-resistant protein 1 inhibitors or substrates was also predicted. As can be seen, none of the peptides were predicted to be Pgp inhibitors. In addition, several of the peptides (with the exception of EQALMGFYGPTEDPNGS, ELAQMGPEGPMGLEDPNGS, DPNGSPYYWKELAQM, EQALMPWIWYWKDPNGS, and PIWWYWKDPNGS) were also predicted to demonstrate very little activity as Pgp substrates, which indicates that the likelihood of these peptides activating PgP and driving efflux is low [
112]. The web server was also used to predict blood-brain barrier (BBB) permeability. All peptides, with the exception of DPNGSPYYYWKELAQM and EQALMGFYGPTEDPNGS, were found to be BBB permeable, which is promising.
5. Conclusions and Future Work
In this study, we utilized a peptide scrambling approach to design 20 biomimetic peptides that comprised both antioxidant and fibrillary inhibition moieties. Of those, 12 peptides were then selected for investigating their antioxidant activity, secondary structures, and binding abilities with two forms of ASyn (pathogenic fibrillar and Lewy body dementia-derived filaments) using computation methods. Our results indicate that the peptides showed free radical scavenging scores between 0.53 and 0.73, with higher values predicted to show higher antioxidant activity. The molecular docking and MD simulations revealed key interactions with the NAC domain as well as with the pre-NAC domain and the appearance of alpha-helical structures over time, particularly with PYYYWKELAQM and ELAQMPYYYWKDPNGS. The docking studies revealed that, in general, the peptides bound to pivotal residues in the NAC domain, including residues such as Ala78, Val77, Ile88, and Gly73, among others. The molecular dynamics simulations further showed stable binding with most peptides, which was further confirmed by MMGBSA analysis, showing a ΔGbind energy between −80 kcal/mol and −118 kcal/mol for the peptides upon binding with the filamentous Lewy body-derived ASyn. Two peptides (PIWWWYWKDPNGS and PYYYYWKELAQM), however, showed a relatively lower ΔGbind (between −50 and −58.7 kcal/mol). On the other hand, PYYYWDKPNGS showed the highest ΔGbind with multichain fibrillar ASyn at −101.8 kcal/mol, while DPNGSPIWWYWKELAQM showed the lowest binding energy at −57.6 kcal/mol. Thus, the binding interactions varied between the filamentous and fibrillar ASyn. Based on the computational results, five peptides were selected for laboratory validation studies. In general, we observed that the peptides induced conformation changes in the ASyn fibrils. The results corroborate the MD simulations. Furthermore, antiparallel beta-sheets were found to reduce over time, with the concurrent appearance of disordered structures and turns, while some of the peptides also showed changes in alpha-helical content. In some cases, the appearance of parallel beta-sheet structures was observed, though to a lesser extent. The results were also confirmed by using thioflavin-T assays, which showed a reduction in fluorescence due to fibrillar ASyn over time. Overall, it appears that PYYYWKDPNGS and ELAQMPYYYWKDPNGS demonstrated both antioxidant activity and a reduction in beta-sheets over time and may be potentially utilized for further investigation into laboratory studies or conjugated with drugs that may mitigate the fibrillation of alpha-synuclein as a potential therapeutic. This work proposes a number of peptides that can be investigated to examine their impact on alpha-synuclein misfolding. Further studies will involve investigating the impact of the peptides in a cellular environment where the internalization of these peptides will be studied, as well as the impact on intra and extracellular ASyn fibrils.