1. Introduction
The EU’s hydrogen strategy and REPowerEU plan have put forward a comprehensive framework to support the uptake of renewable and low-carbon hydrogen to help decarbonize the EU in a cost-effective way and reduce its dependence on imported fossil fuels [
1]. In this scenario, fuel cells and electrolyzers have the potential to meet the world’s current energy needs due to their efficiency, stability, and wide range of applications, from stationary to transportation sectors [
2]. Electrochemical devices based on proton-conductive ceramic materials have gained increasing attention as an appealing solution due to the possibility of working efficiently at lower (intermediate) temperatures [
3]. Recent studies have fully demonstrated the possibility of applying ceramic proton conductors in protonic ceramic fuel cells (PCFCs) and electrolyzers (PCEs) [
4,
5,
6,
7,
8,
9,
10], membrane rectors [
11,
12,
13,
14], hydrogen purification devices [
15,
16,
17,
18], and hydrogen sensors [
19,
20].
Perovskite oxides are among the most promising ceramic proton conductors. Ideal perovskites have the general formula ABO3, where the A-sites are typically occupied by larger cations than the B-sites and analogous in size to the O-site anions. The ABO3 structure can be considered a face-centered cubic (fcc) lattice with the A atoms at the corners and the O atoms on the faces. The B atom is located at the center of the lattice. The structure of perovskite is formed by a three-dimensionally connected system of BO6 octahedra and AO12 cube-octahedra at the edges of the B-centered cubic lattice. In proton-conductive perovskites, a trivalent cation is generally partially substituted into the B-site to increase the oxygen vacancy concentration in the composite. The general formula of these doped perovskites can be written as AB1−xMxO3−δ, where x is less than the upper limit of its solid solution formation range (usually less than 0.2), and δ denotes the number of oxygen deficiencies per unit formula of the perovskite.
Perovskite materials, such as doped BaCeO
3 (BCO), BaZrO
3 (BZO), and their solid-solution BaCe
xZr
yY
1−xyO
3−δ (BCZY)- and BaCe
xZr
yY
zYb
1−x−y−zO
3−δ (BCZYYb)-type ceramics, have been extensively studied thanks to the good compromise among chemical stability, thermomechanical resistance, and high protonic conductivity. They are expected to play a pivotal role in the near-term development of performant, low-cost devices for the H
2 economy [
3].
The proton conductivity of these systems relies on the defect formation and distribution in the perovskite lattice, but also on external factors, such as temperature, partial pressure, and the nature of the atmosphere. Protons are formed in the water vapor or hydrogen-containing environment at high temperatures according to the following reactions (1)–(3):
where H• stands for a proton. Moreover, it was found that protons bond with oxygen atoms to form substitutional hydroxyls, as follows [21]:
which are the main reactions responsible for proton conduction. Thus, for protonic conduction, the material first needs to incorporate protons through hydroxide formation, and then the transport mechanism takes place by interstitial proton hopping through intra-octahedral O sites in the presence of oxygen vacancies [22]. Proton migration through the lattice via the hopping process includes the fast rotation and reorientation of the proton, which implies the localization of the proton in the vicinity of an oxygen ion, followed by its transfer to the next ion [23,24].
Since their discovery in the 1980s, several efforts have been made in the development of proton-conducting ceramic materials [
25]. However, the manufacturing of these devices has faced several issues due to the poor sinterability of cerate and zirconate perovskites, which requires high temperatures of up to 1700 °C and long processing times (4–40 h) to be fully densified (
Table 1). The obtainment of hermetic gas-tight ceramic electrolytes is a key step to enhancing the proton conductivity and durability of PC-SOCs/SOEs [
26]. Moreover, the refractive nature of this class of materials (especially BaZrO
3-type ceramics) leads to the generation of a higher density of grain boundaries, which are generally more resistive compared to the bulk due to the existence of a space charge depletion layer that generates the so-called “grain boundary blocking effect” [
27]. Recent studies have revealed that the proper optimization of the dopant concentration and processing methodology (manufacturing and sintering treatment) can effectively suppress the blocking effect, thus providing high proton conductivity [
28].
An important aspect to consider when sintering BaCeO
3-BaZrO
3-type perovskites is their poor thermal stability at high temperatures. The first studies on this phenomenon were conducted by D. Shima and co-workers, who investigated the influence of cation non-stoichiometry on the conductivity of doped and undoped BaCeO
3 ceramics [
29]. They found that small changes in Ba content have a dramatic effect on proton conductivity and hypothesized that severe barium loss occurs during high-temperature sintering. This was further confirmed by Glockner et al. [
30], who performed atomistic simulations of defect formation in BaCeO
3 compounds, revealing the favorable formation energy for Ba and O vacancy pairs, which could result in the loss of BaO at very high temperatures. Experimental studies revealed similar behavior for the more stable, doped and undoped BaZrO
3 ceramics [
31,
32] and BaCeO
3-BaZrO
3 solid solutions [
33,
34,
35,
36].
Table 1.
Sintering conditions and relative density of BaCexY1−xO3–δ (BCY), BaZrxY1−xO3–δ (BZY), BaCexZryY1−x−yO3–δ (BCZY), and BaCexZryYzYb1−x−y−zO3−δ (BCZYYb) ceramics considering different powder synthesis methods, such as solid-state synthesis and wet chemical approaches; values in bold indicate that sintering additives are used.
Table 1.
Sintering conditions and relative density of BaCexY1−xO3–δ (BCY), BaZrxY1−xO3–δ (BZY), BaCexZryY1−x−yO3–δ (BCZY), and BaCexZryYzYb1−x−y−zO3−δ (BCZYYb) ceramics considering different powder synthesis methods, such as solid-state synthesis and wet chemical approaches; values in bold indicate that sintering additives are used.
| Material | Synthesis Route | Sintering
Temperature (°C) | Sintering Time (h) | Relative Density (%) | Ref. |
|---|
| BaCexY1−xO3−δ | Solid-state synthesis | 1450 | 40 | 90 | [37] |
| 1550 | 10 | 92 | [38] |
| 1675 | 10 | 90 | [39] |
| 1500 | 6 | 70 | [40] |
| Wet chemical approaches | 1500 | 6 | 85 | [40] |
| 1500 | 6 | 95 | [40] |
| 1350 | 10 | 74 | [41] |
| 1250 | 10 | 63 | [41] |
| 1650 | 4 | 90 | [42] |
| 1250 | 10 | 91 | [43] |
| 1400 | 10 | 96 | [44] |
| BaZrxY1−xO3−δ | Solid-state synthesis | 1535 | 12 | 94 | [45] |
| 1325 | 20 | 96 | [46] |
| 1600 | 24 | 94 | [47] |
| 1500 | 24 | 30 | [48] |
| 1485 | 12 | 94 | [45] |
| Wet chemical approaches | 1300 | 4 | 60 | [49] |
| 1700 | 12 | 90 | [50] |
| 1600 | 5 | 79 | [51] |
| 1600 | 12 | 91 | [52] |
| 1500 | 10 | 90 | [53] |
| 1700 | 4 | 85 | [49] |
| BaCexZryY1−x−yO3−δ | Solid-state synthesis | 1400 | 8 | 97 | [54] |
| 1600 | 10 | 97 | [55] |
| 1600 | 24 | 96 | [55] |
| Wet chemical approaches | 1450 | 5 | 80 | [56] |
| 1450 | 5 | 96 | [56] |
| 1550 | 4 | 90 | [57] |
| 1450 | 10 | 89 | [36] |
| 1450 | 5 | 88 | [58] |
| BaCexZryYzYb1−x−y−zO3−δ | Solid-state synthesis | 1550 | 10 | 97 | [59] |
| 1550 | 10 | 97 | [60] |
| 1450 | 3 | 98 | [61] |
| Wet chemical approaches | 1400 | 6 | 95 | [62] |
| 1400 | 10 | 98 | [63] |
| 1400 | 5 | 97 | [64] |
To overcome this issue, several efforts have been made to produce these ceramic proton conductors in mild conditions. As shown in
Table 1, the use of powders produced by wet chemical approaches, such as co-precipitation and combustion synthesis, generally helps in lowering the sintering temperature and/or time with respect to the use of powders synthesized by the conventional solid-state methodology [
65]. This is mainly due to the finer and more reactive nature of the powder produced by wet chemical strategies. Furthermore, the use of sintering aids such as ZnO, NiO, and CuO has been extensively studied and has proven to be effective in reducing both the sintering temperature and processing time, regardless of the synthetic approach [
66]. In this case, the formation of a low-temperature melting phase is responsible for increasing the ion diffusion and the formation of defects, which both help mass transport and densification [
67]. However, the sintering conditions are still quite harsh (with temperatures higher than 1300 °C) and highly energy-consuming. Moreover, the possible detrimental effect on the conductivity of proton-conductive ceramics due to the presence of residual sintering aids on the grain boundaries is still under debate [
68].
Regardless of the sintering treatment, one well-consolidated strategy to repress barium evaporation from BaCeO
3-BaZrO
3 ceramics is to control the sintering atmosphere by introducing sacrificial Ba-containing powders (the so-called “pack”) or pellets in the sintering setup. In this way, it is possible to finely control the composition of the perovskite phase, even at a high temperature of about 1550 °C [
26,
34,
69]. Otherwise, since BaO evaporation starts from the surface and only a small fraction of the superficial part of the material undergoes compositional changes, light mechanical abrasion of the surface of the sintered ceramics is generally carried out. However, this approach is suitable only at the lab scale for relatively thick devices; therefore, conventional PC-SOC/SOE devices produced with thin-film electrolytes and at medium–large scales have been excluded.
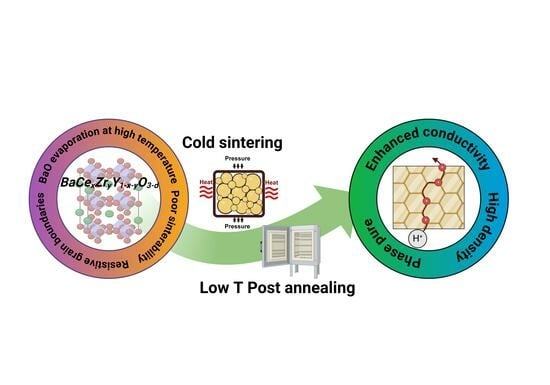
Although several improvements have been made in manufacturing proton-conductive ceramic materials, producing highly efficient devices in mild conditions is still a great challenge. Moreover, recent studies revealed the formation of Stacking Fault defects in BZY- or BCZY-type ceramics when conventionally sintered, and these defects detrimentally affect both the conductivity and mechanical properties of the final device [
70]. Exploring novel sintering technologies could be an effective way to achieve technological advances in the production of highly efficient proton conductor devices.
Among others, the cold sintering process (CSP) presents unique advantages in terms of material properties, flexibility, and costs, and it is considered one of the most promising ways to obtain ceramic proton conductors in mild conditions. However, to the best of our knowledge, no comprehensive article has been published that provides a complete overview of the CSP findings and advancements in BaCeO3/BaZrO3-based proton conductors. For these reasons, the aim of this review is to provide an overview of the theory and technological advancements related to the cold sintering process. A comparison of various studies on BaCeO3/BaZrO3-based perovskites is also considered, highlighting the main challenges and advantages in the process application. The future trends and prospects of the method in this field are addressed as well.
2. Cold Sintering Process
Cold sintering is a geologically inspired sintering process where densification is driven by mechanical–chemical effects (pressure solution creep or dissolution–precipitation creep) in synergy with chemical effects [
71].
Pressure solution is a fluid-assisted, stress-driven mass transport enabled by chemical potential gradients, typically associated with the upper Earth’s crust rock’s densification and deformation [
72]. Despite being a very slow process in nature, it is strongly accelerated in fine-grained materials and can occur not only in the presence of aqueous solutions but also with a partial eutectic melt or any intergranular solution phase, as long as the grain boundaries are wetted [
73].
During cold sintering, materials are densified in the presence of a transient liquid phase, a compound that promotes dissolution and precipitation reactions and subsequent compaction with the aid of an external uniaxial applied pressure (100–1000 MPa), and consolidation is carried out between room temperature and above the boiling point of the liquid (typically < 350 °C). The time required to obtain high-density ceramic materials is typically less than one hour [
74].
A schematic representation of the cold sintering process and the thermocompression apparatus employed is shown in
Figure 1. First, an appropriate amount of the transient phase (liquid or solid) is introduced to the particle ensembles. When the transient phase is a liquid, this first stage enables the local dissolution of the sharp surfaces of the particles, and the liquid acts as a lubricant to promote particle rearrangement and sliding. With the assistance of an applied external pressure, the phase fills the interstitial space among particles, providing the initial particle compaction. The temperature is raised to the boiling point (or melting point if solid) of the transient phase, enhancing the solubility of ceramics and the formation of a supersaturated environment through the evaporation of the liquid phase from the pellet and the die, which is not perfectly sealed.
The presence of an external uniaxial load provides the strain that promotes mass transport and densification. The driving force during the process is the chemical potential gradients, from highly constrained areas with enhanced dissolution and high chemical potential to weakly constrained areas at particle surfaces with a lower chemical potential, through the liquid film (
Figure 2) [
75].
A wide range of materials have been successfully densified through the CSP, including structural ceramics [
73,
76,
77,
78], microwave dielectrics [
79,
80,
81,
82,
83], ferroelectrics [
84,
85,
86,
87,
88], piezoelectrics [
89,
90,
91,
92], Li-ion cathodes [
93,
94,
95,
96,
97], transparent ceramics [
98,
99,
100,
101,
102,
103], solid-state electrolytes [
104,
105,
106,
107,
108,
109,
110,
111,
112,
113,
114,
115], thermoelectric materials [
116,
117,
118,
119], ceramics for electronics [
120,
121,
122] and environmental applications [
123,
124,
125], supercapacitors [
126], magnetic ceramics [
93,
127,
128,
129,
130,
131], bioceramics [
132,
133,
134,
135,
136], bulk van der Waals materials (MXene, MoS
2, reduced graphene oxide, etc.) [
137], and metals [
138,
139]. Moreover, the mild sintering conditions made possible the co-sintering of dissimilar materials with large differences in processing temperature windows, such as new ceramic–polymer composites [
140,
141,
142,
143,
144,
145,
146], and thermodynamically unstable phases [
147]. The CSP is suitable for densifying materials not only in the form of powders but also in green ceramic parts (generally after organic removal) and multilayers. The conventional sintering and assembly of a working device require several steps with repeated heating, including forming (e.g., tape casting), multilayer assembly, firing, metallization of electrodes, and integration with a substrate. The CSP allows, in a single step, the integration of organic and inorganic components, and it has the potential to surpass conventional processing routes by offering unprecedented “all in one step” solutions to manufacture devices.
Recently, the CSP has been applied to the densification of barium cerate and zirconate ceramics. Cold sintering was successfully demonstrated for the fabrication of BZY- [
112,
148] and BCZY-based proton-conductive electrolytes [
110,
111,
113,
149,
150], as well as cer–met anodes [
151], for PC-SOC/SOE devices at the lab scale.
However, although several years have passed since the first patent in 2016, the studies related to cold sintering have mainly been devoted to the densification of powders. Despite the ease of operation and the simplicity of the equipment required for the cold sintering process (i.e., a thermocompression die apparatus), there are several parameters to be taken into account when considering the CSP (
Figure 3), and the obtainment of compact ceramics with high density, phase purity, and engineered microstructure is not trivial. Further insight into the mechanisms involved during cold sintering is provided in the following paragraph.
3. Cold Sintering Mechanisms
The cold sintering kinetics and mass transport mechanisms are in the early stages of investigation, and a complete analytical model for a deep understanding of the sintering process is still lacking. However, experimental and theoretical investigations allow the identification of two main stages during the CSP (
Figure 4). The following explanation does not take into account solid transient phases such as hydrated salts or eutectic mixtures.
Stage I involves interactions between ceramic particles and a liquid under the application of uniaxial pressure. When a solid substance is in contact with a liquid, four different phenomena can be observed: (i) dissolution, (ii) chemical reactions, (iii) absorption limited to the surface, (iv) electrostatic/steric repulsion/wetting. The first two are considered beneficial in promoting consolidation in the CSP [
153]. Frequently, the liquid fraction is small (<10%), and consolidation is achieved by partial solubilization [
154]. During Stage I, the total volume of the system slightly decreases because of drying, causing material shrinkage. This phenomenon takes place until the solid particles form a rigid skeleton, and then Stage II starts. This stage is predominated by pressure- and temperature-assisted dissolution and precipitation events that are driven by local and global gradients within the pellet die [
155], promoting rapid densification through the pressure solution creep (PSC) mechanism. PSC is a dissolution–precipitation process that relies on the transport of matter from the contact between touching particles to the surrounding liquid phase and eventually to nearby non-contacting surfaces. Such transport locally decreases the distance between the particle centers, enabling global shrinkage and densification [
73]. This mechanism is driven by chemical potential gradients, from highly constrained areas with enhanced dissolution and high chemical potential to weakly constrained areas at particle surfaces with a lower chemical potential, through atomically thin liquid films at the contacting particle surfaces [
72]. At this stage, the system is likely to respond in at least three possible routes [
155]: (1) the heterogeneous nucleation of dissolved species, a process that minimizes free energy by reducing the surface area and by ultimately replacing the solid–liquid interface with solid–solid grain boundary interfaces; (2) homogeneous nucleation, where new crystals precipitate in the interstitial space between grains [
156]; (3) a step-wise transition, whereby a metastable glass phase or intermediate compound is formed to bridge the initial solutes and final product [
157]. Recent studies have evidenced that, during this stage, most of the fluid is extracted from the pellet die [
158]. At the end, materials are, in general, almost fully densified and are then subjected to constant temperature and pressure for several minutes, where the prevalent effect is grain growth, occurring with a relatively slow kinetic process [
159].
During these stages, the transient chemistry, the sintering temperature and rate, uniaxial pressure, and dwell time (t) are the main densification process variables. Depending on the chemistry of the particle–solvent interaction, either congruent or incongruent dissolution on the surface can occur [
154,
160]. In the former case, the material dissolves into the solvent with a homogeneous chemical stoichiometry before mass transport and precipitation [
127], and the resulting liquid solution enables the hydrothermal environment for precipitation and crystal growth [
71]. The particles then precipitate from the supersaturated solution when a strain, temperature, or curvature gradient is encountered, or due to the evaporation of the solvent phase through the die [
160]. Typically, when evaporation is fast, solute gelation and the subsequent formation of a disordered phase within the grain boundaries are observed [
79,
122,
161].
Incongruent dissolution results instead in a material that has a different composition/stoichiometry when compared with the parent phase [
71]. Here, ions with higher dissolution kinetics are leached out before those with slower dissolution rates, leading to the formation of unwanted secondary phases and hindering densification. Moreover, these byproducts are generally deleterious to the final properties [
157,
162,
163]. The formation of undesired phases can be mitigated by saturating the transient phase with cations with higher solubility in the selected solvent [
110,
112,
113,
164] to slow down the dissolution kinetic of these ions, reducing the surface passivation process and allowing the dissolution–precipitation reactions to proceed. However, when incongruent dissolution occurs, it is almost impossible to completely prevent the formation of crystalline or amorphous/glassy phases within the sample. In such cases, a post-annealing (PA) step is required to induce solid-state reactions between crystalline impurities or speed up the metastable glass-to-crystalline phase transition. In these conditions, epitaxial crystal growth takes place via mass transport since the corresponding ionic species, atomic clusters, or ligands in the glass phase precipitate onto the surface of the crystallites (recrystallization) [
71,
165]. Post-annealing is often employed even when no secondary phases are present to increase the grain size, which is generally limited during cold sintering. It should be mentioned that, in some cases, the formation of secondary phases during cold sintering is desired and can be an effective way to engineer the grain boundaries of the material [
149].
It has recently been shown that incongruent dissolution can be avoided by the use of more chemically active solvents, such as molten hydroxides and salts [
162,
166,
167] or chelating agents like acetylacetonates [
93]. This means that the selection of an effective transient chemical phase is not trivial and is probably the most influential parameter affecting the process.
It is worth mentioning that, in some cases, the transient phase was found to not dissolve ceramic particles (the case of negligible dissolution), but densification can also occur during cold sintering if soluble salts are mixed with the ceramics [
168,
169]. In such cases, the soluble phases lubricate the insoluble ceramics, resulting in compaction via plastic deformation or subcritical crack growth processes [
170], but generally, a post-annealing step is required to further improve densification.
5. The Application of the Cold Sintering Process to Ceramic Proton Conductors
The feasibility of performing the CSP on barium cerate and/or zirconate ceramics has been demonstrated over the years, and the results obtained up to now are reported in
Table 3. The main strategy adopted to cold-sinter BCZY- and BZY-type ceramics involves the introduction of an aqueous transient phase into the as-synthetized ceramic powders.
Kindelmann et al. [
148] cold-sintered BaZr
0.8Y
0.2O
3−δ (BZY20) ceramics starting from BaCO
3, ZrO
2, and Y
2O
3 precursors planetary ball milled in isopropanol and calcined at 1175 °C for 3 h in air. CSP experiments were performed in a FAST/SPS machine in the presence of 5 wt% deionized water as the transient phase. Experiments were carried out at temperatures between 150 and 250 °C, applying 400 MPa of uniaxial pressure and a dwelling time between 10 and 60 min. The relative density increased from 80 to 85 and 87% when increasing the temperature from 150 to 200 and 250 °C, respectively. At the considered maximum temperature of 250 °C and 400 MPa, cold-sintered BZY20 exhibits a heterogeneous microstructure with the presence of residual pores and secondary phases. When cold sintering is performed in BZY-type compounds, densification is driven by the formation of Y(OH)
3 species [
148] as follows:
Since cold sintering is generally performed in an ambient atmosphere (i.e., CO
2 is present in the reaction environment), barium hydroxide rapidly reacts with the dissolved CO
2 to form the more thermodynamically stable barium carbonate:
It is worth mentioning that BaCO
3 was also found when cold sintering was performed in a controlled atmosphere [
148], but the presence of BaCO
3 in the starting powders could be an explanation. Moreover, barium-based compounds react easily with the atmospheric CO
2 according to
Interestingly, Kindelman et al. [
148] pointed out that pressure solution creep mechanisms are not activated by the presence of BaCO
3 alone, since no cold sintering activity was observed in BaZrO
3 ceramics processed in the same conditions as BZY.
Considering the high amount of secondary phase produced, a post-annealing treatment (PA) was required to improve the densification degree and phase purity. Here, a two-step approach was considered, and a thermal post-treatment between 900 and 1100 °C in the same FAST/SPS apparatus imposing 100 MPa of uniaxial pressure was performed directly after cold sintering. This innovative approach allowed the formation of highly dense (≈95%) BaZr0.8Y0.2O3−δ with nano-sized microstructure but with evidence of impurities such as Y2O3 and BaCO3 that detrimentally affect the proton conductivity of the produced material. The conditions employed for the PA treatment should be carefully evaluated based on the thermodynamic stability of the compounds formed during cold sintering.
Zhao et al. [
112] cold-sintered BZY20 ceramics starting from as-synthesized powders moisturized with 20 wt. % of an aqueous PVA solution (3 wt. %) at 180 °C and 400 MPa for 1 h. A high green density of up to 76% was achieved after cold sintering, which is about 20% higher than the relative density obtained after traditional dry pressing. However, when a diluted polyvinyl alcohol (PVA) solution is used instead of pure water [
112], some polymeric residue could remain in the green pellet since the boiling point of PVA is around 340 °C (melting point 200 °C). X-ray diffraction analysis revealed the presence of BaCO
3 impurities, but no evidence of Y(OH)
3 was detected, likely due to its low concentration or poor crystallinity, making it difficult to detect using this technique. Here, a post-annealing treatment at 1500 °C for 12 h was performed to reintroduce these secondary phases into the crystalline lattice. In this way, highly dense (94%) and pure-phase BZY20 ceramics were obtained, showing a homogeneous microstructure composed of micrometric grains and electrochemical properties comparable to those of traditionally sintered samples thermally treated at higher temperatures of up to 1700 °C.
Another feature of the cold sintering process is the possibility of producing the desired ceramic phase in situ (i.e., directly in the sintering environment) through reactive cold sintering. Shen et al. [
199] obtained pure BaZrO
3 ceramics with an acceptable density (≈92%) after treating Ba(OH)
2·8H
2O and Zr(OH)
4 precursors at 300 °C and 500 MPa for 2 h. Here, densification is carried out by the dehydration of Ba(OH)
2·8H
2O, followed by the dissolution of barium hydroxide and the formation of an alkaline environment, as follows:
As the pH increases, the solubility of zirconium hydroxide rises, and BaZrO3 nuclei are rapidly formed with increasing temperature, while the holding time promotes the growth and maturation of BaZrO3 particles. Although no information is provided regarding the performance of the reactive cold-sintered BaZrO3 ceramics, this method can be an interesting way to obtain dense and pure proton-conductive perovskites in one step, avoiding post-annealing treatments.
Regarding Ce-containing compositions (i.e., BCZY-type), Thabet et al. [
110,
111] cold-sintered as-synthesized BaCe
0.8Zr
0.1Y
0.1O
3−δ powders obtained by the nitrate–glycine method at temperatures from 120 to 180 °C for 30 min, at applied pressures ranging from 125 to 500 MPa, and with different amounts of water. For temperatures lower than 160 °C, the obtained density was relatively low, about 70%, while the applied pressure had a minor effect. A high green density of about 83% was obtained by increasing the temperature up to 180 °C while applying 375 MPa of pressure in the presence of the 5 wt. % of water. After cold sintering, a post-annealing treatment was performed at 1200 °C for 10 h. In this way, BCZY electrolytes with densities of up to 94% were produced, showing a remarkable proton conductivity of 2.5 × 10
−2 S cm at 600 °C. The authors associated the high total conductivity with increased grain boundary conduction compared to traditionally sintered samples. The addition of higher amounts of water during cold sintering (10–20 wt. %) was found to detrimentally affect the densification and conductivity of the material due to the formation of a larger number of impurities, such as BaCO
3 at the grain boundaries, that remain after post-annealing.
Kindelmann et al. [
113] studied the possibility of applying cold sintering to densify BaCe
0.2Zr
0.7Y
0.1O
3−δ ceramics by controlling the phase composition of the starting powders. Here, ceramic precursors optimized for solid-state reactive sintering composed of BaCO
3, CeO
2, ZrO
2, and Y
2O
3 pre-calcined at 1100 °C for 1 h were added with 0.5 wt% of NiO, recalcined at 1300 °C for 1 to 20 h, planetary milled in ethanol, and finally used as the starting mixture for the cold sintering experiments after sieving at 100 µm. Ceramic powders were moisturized with 5 wt. % of water and treated at 350 °C and 400 MPa for 5 min in a FAST/SPS apparatus. The authors found that a higher green density of about 80% was obtained with a shorter calcination treatment of 1–5 h since, in these conditions, the starting powders are composed of a Ce-rich and a Zr-rich BCZY phase. Longer calcination times led to the formation of the target pure-phase BCZY, decreasing the cold sintering activity. For BCZY-type materials, the driving force toward dissolution and precipitation events during cold sintering was found to be favored by the use of a Ce-rich BCZY phase as the starting powder. Water preferentially dissolves barium from the perovskite structure, followed by cerium and yttrium, leading to the formation of a Zr-rich BCZY phase, barium hydroxide, and ceria/Y-doped cerium oxide compounds, as follows:
As explained above, BaCO
3 is also produced as a consequence of reaction (9). These byproducts that formed during cold sintering had low strength, easing particle rotation and sliding under high mechanical pressures, enabling densification [
149]. However, a post-annealing step was always required to induce solid-state reactions between impurities to obtain highly dense and phase-pure ceramics. After a post-annealing step at 1300 °C for 10 h, a phase-pure BCZY electrolyte showing a 96% relative density was obtained with nanometric grain size and an exceptional proton conductivity of 4 × 10
−2 S cm at 600 °C, which is among the highest reported in the literature for BCZY-type proton conductors [
25]. This is related to the unique characteristics offered by the cold sintering treatment: as the process undergoes dissolution and precipitation reactions, secondary phases such as BaCO
3 and Y-doped CeO
2 are produced in the grain boundary (GB) region, and during the post-annealing treatment, they react together with the BCZY phase, changing the grain boundary composition (
Figure 5a,b). This leads to cationic segregations (and enrichment) in the GB region (
Figure 5c–e), which causes a huge drop in the grain boundary resistivity and, consequently, an increase in total conductivity.
Table 3.
Cold sintering parameters and related post-annealing (PA) conditions for the processing of barium cerate and/or zirconate ceramics reported in the literature: composition, particle size (d50), the type and amount of transient phase, temperature (T), heating rate (R), pressure (P), dwell time (t), the relative density of the as-cold-sintered and post-annealed samples, and the total conductivity values (σT) of the post-annealed specimens, measured in wet air at 600 °C.
Table 3.
Cold sintering parameters and related post-annealing (PA) conditions for the processing of barium cerate and/or zirconate ceramics reported in the literature: composition, particle size (d50), the type and amount of transient phase, temperature (T), heating rate (R), pressure (P), dwell time (t), the relative density of the as-cold-sintered and post-annealed samples, and the total conductivity values (σT) of the post-annealed specimens, measured in wet air at 600 °C.
| Material | Starting Powders’ Characteristics | Transient Phase | Cold Sintering Parameters | PA | Density (%) | σT,600°C Wet Air (S/cm) | Ref. |
|---|
| Composition | d50 (µm) | Type | wt% | T (°C) | R (°C/min) | P (MPa) | t (h) | T (°C) | t (h) | CSP | PA | | |
|---|
| BaZr0.8Y0.2O3−δ | BaZr0.8Y0.2O3−δ synthetized by a modified Pechini method [200] | n.r. | 3 wt % PVA(aq) | 20 | 180 | 10 | 400 | 1 | 1500 | 12 | 76 | 94 | 0.003 | [112] |
| $ BaZr0.8Y0.2O3−δ | Mixture of BaCO3, ZrO2, and Y2O3 ball milled in isopropanol and calcined at 1175 °C for 3 h | <0.5 | H2O | 5 | 250 | 20 | 400 | 0.1 | 1100 # | 0.1 | 87 | 95 | 1 × 10−5 | [148] |
| * BaZrO3 | Ba(OH)2·8H2O | 0.5 | Structural
water | 350 | 20 | 500 | 2 | / | 92 | / | n.r. | [199] |
| Zr(OH)4 | 0.02–0.1 |
| Ba/Zr = 1.15 | / |
| BaCe0.8Zr0.1Y0.1O3−δ | BaCe0.8Zr0.1Y0.1O3−δ obtained by nitrate–glycine method [201] | 23.3 | H2O | 5 | 180 | 5 | 375 | 0.5 | 1200 | 10 | 83 | 94 | 0.025 | [110,111] |
| BaCe0.7Zr0.2Y0.1O3−δ | Commercial BaCe0.7Zr0.2Y0.1O3−δ with bimodal particle size (Cerpotech) | 0.8–2; 5–11 | H2O | 5 | 180 | 5 | 375 | 1 | 1600 | 15 | n.r. | 91 | 0.004 | [150] |
| $ BaCe0.2Zr0.7Y0.1O3−δ | Mixture of BCY, BZY, BaCO3, and (Zr,Y)O2 + 0.5 wt. % NiO [202] calcined at 1300 °C for 5 h, milled, and sieved at 100 µm | 0.6 | H2O | 5 | 350 | 20 | 400 | 0.1 | 1300 | 10 | 80 | 96 | 0.04 | [113,149] |
As previously mentioned, the composition and characteristics of the starting powders (size, morphology, etc.) can drastically influence the densification degree during cold sintering (and PA) and the final electrochemical properties. For example, Castellani et al. [
150] obtained ≈91% dense BaCe
0.7Zr
0.2Y
0.1O
3−δ ceramics after cold sintering commercial BCZY powders at 180 °C and 375 MPa for 60 min in the presence of 5 wt. % of water, followed by a post-annealing treatment at 1600 °C for 15 h. Similar or even better results in terms of densification degree and electrochemical properties can be obtained with conventional approaches (
Table 1), as the proton conductivity was found to be one order of magnitude lower compared to traditionally sintered devices. These studies clearly pointed out that the composition of the starting ceramic powders has to be carefully evaluated to properly trigger cold sintering reactions and tune the grain boundary composition of the material.
Achieving pure and dense BCZY-based ceramics under mild conditions is the primary requirement for cold sintering to be considered a viable method for processing proton-conductive ceramics. As explained in the Introduction, this class of materials is widely used in proton-conductive solid oxide cells and electrolyzers, which are multilayer-structured devices. The only study regarding the feasibility of performing cold sintering in the production of anode-supported proton-conductive half-cells was recently reported by Kindelmann et al. [
203]. In this case, the die was filled in a multi-step manner, pouring first a thicker layer (≈2.0 mm) of the composite BaCe
0.2Zr
0.7Y
0.1O
3−δ-NiO (BCZY:NiO = 1:1) powders and then a thinner film of pure BCZY (≈0.7 mm). The bilayer was then pre-compacted, and the disc was humidified with 5 wt% of water before being cold-sintered at 350 °C and 400 MPa for 5 min in a FAST/SPS setup. In these conditions, the dissolution of NiO was negligible, and cold sintering was promoted by the BCZY phase, as explained above, leading to a green density of up to 80%. The half-cells were then post-annealed at 1300 °C for 10 h in air and subsequently treated at 900 °C in H
2/Ar to reduce NiO to metallic nickel (
Figure 6).
A good contact between the two layers and a high densification degree were obtained; however, the microstructure of the composite needs further optimization. Moreover, the thickness of the dense electrolyte layer was ≈0.3 mm thick, and further work should be carried out to produce thin layers of ≈0.02 mm that can be effectively applied in protonic solid oxide cells.