The relationship between fibromyalgia and inflammation represents a multifaceted and dynamic area of investigation, highlighting the sophisticated interplay between the immune system and the nervous system [
120]. While fibromyalgia has traditionally been classified as a disorder primarily characterized by CNS sensitization, recent evidence increasingly emphasizes the role of inflammation in its pathophysiology [
121,
122]. The inflammatory milieu is thought to exacerbate the increased sensitivity to pain that typifies this condition, because it promotes peripheral nociceptor sensitization while amplifying central sensitization processes [
121]. Furthermore, neuroinflammation (the interplay between immune cells and the nervous system) may worsen symptoms, creating a feedback loop that perpetuates pain [
122].
Although the complexity of this relationship is evident, it underscores the need for a multidisciplinary approach to fully understand this connection. Given these findings, researchers and clinicians must work together to uncover the underlying mechanisms.
3.1. Peripheral Inflammation and Fibromyalgia
The recent evidence underscores the pivotal role of peripheral inflammation in the pathophysiology of fibromyalgia, indicating that inflammation is not simply a result of fibromyalgia but a fundamental factor driving its development and ongoing persistence [
121]. Some studies, employing the ELISA method, have demonstrated that fibromyalgia patients often exhibit elevated levels of inflammatory markers (
Table 2), including C-reactive protein (CRP), which serves as a good indicator of low-grade systemic inflammation [
123,
124,
125]. However, not all individuals with fibromyalgia manifest elevated CRP levels; in fact, the extent of elevation can differ markedly across patients. Some studies have indicated that CRP concentrations in individuals with fibromyalgia may be influenced by comorbid conditions, such as obesity or concurrent inflammatory disorders, highly prevalent within this cohort [
126,
127]. This inflammatory state can begin a catalytic effect, amplifying the painful symptoms that characterize fibromyalgia and potentially exacerbating the overall burden of the condition [
128]. Such complexity requires further investigation, as understanding these relationships is vital for developing effective treatment strategies. CCL2, a chemokine involved in the inflammation process [
129], has been identified in the plasma of patients with fibromyalgia using the ELISA method [
9].
On the other hand, some studies have identified the interferon gene signature in fibromyalgia patients. This signature is characterized by the increased expression of interferon-regulated genes in peripheral B cells, as assessed by RNA sequencing and RT-qPCR. These genes, including
S100A8,
S100A9,
VCAM,
CD163,
SERPINA1, and
ANXA1, are involved in immune response and inflammation [
14]. The elevated expression of these genes demonstrates that fibromyalgia might involve an autoimmune-like aspect, with the immune system targeting the body’s own tissues [
139].
A recent publication showed the proteomes of plasma, serum, and saliva in healthy individuals and fibromyalgia patients. The most significant proteins identified in patients with fibromyalgia included transferrin; α-, β-, and γ-fibrinogen chains; profilin-1; transaldolase; PGAM1; apolipoprotein-C3; complement C4A and C1QC; immunoglobulin components; and acute phase reactants [
130]. Some of these are implicated in the maintenance of chronic low-grade inflammation [
140,
141,
142,
143]. Moreover, increased levels of inflammatory serum proteins, including IL-8, IL-37, AXIN1, and SIRT2, have been identified through proteomics and ELISA as correlates of fibromyalgia symptom severity [
14,
144]. IL-8 and IL-37 participate in the recruitment of some immune cells to inflammation sites. Consequently, its elevated levels show a highly active inflammatory response in patients with fibromyalgia [
145,
146]. Similarly, AXIN1 and SIRT2 are linked to immune regulation and cellular stress and have been linked to the severity of pain, fatigue, and some other symptoms in fibromyalgia [
147,
148]. Other investigations that analyzed plasma proteins using ELISA have reported elevated levels (pro-inflammatory cytokines) alongside reductions in IL-4 and IL-13 (anti-inflammatory cytokines), thereby inducing the activation of various immune cells, including mast cells [
131,
132,
133,
134,
135]. In this regard, fibromyalgia patients have displayed, as assessed by flow cytometry, an increased neutrophil/lymphocyte ratio and alterations in several T lymphocyte subpopulations, including CD4
+ T cells and NKT cells [
149,
150]. All the aforementioned biomarkers provide important insights into the inflammatory processes involved in fibromyalgia, thereby serving as potential targets for the development of future diagnostic tools [
151,
152].
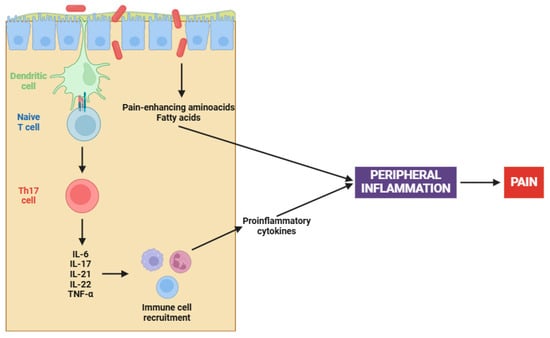
On the other hand, the complex interaction between gut microbiota and fibromyalgia has emerged as a central focus in elucidating the pathophysiology of this chronic condition [
153,
154]. Recent studies have illuminated a compelling causal nexus between altered gut microbiota and fibromyalgia symptoms, particularly in relation to peripheral sensitization [
155]. Pioneering research has demonstrated that fecal microbiota transplantation (FMT) from patients with fibromyalgia into germ-free mice induces pain hypersensitivity; however, transplantation from healthy individuals has been ineffective in reproducing this phenomenon [
156,
157]. Certainly, this involves an increase in pro-inflammatory cytokines (e.g., IL-17 and TNF-α) and the activation of monocytes and lymphocytes, both contributing to the peripheral sensitization process (
Figure 1) [
156,
157].
The altered microbiota in fibromyalgia patients is characterized by imbalances in certain bacterial species, such as
Flavonifractor plautii,
Parabacteroides merdae, and
Faecalibacterium prausnitzii [
158]. Apart from cytokine participation, microbial imbalances contribute to peripheral sensitization through several mechanisms, including the synthesis of pain-enhancing amino acids (e.g., glutamate), alterations in fat metabolism, and changes in bile acid production [
159]. Chronic immune activation, when coupled with an altered gut microbiota, results in increased intestinal permeability. This ultimately leads to further activation of the immune system and initiates an inflammatory cascade that stimulates nociceptive signals [
156,
157].
The connection between peripheral inflammation and fibromyalgia extends beyond pain perception; it also notably influences the comorbid conditions commonly seen in individuals with fibromyalgia [
160,
161]. Numerous conditions, including inflammatory arthritis, chronic spontaneous urticaria (CSU), and functional bowel disorders (FBD), are usually encountered alongside fibromyalgia [
160,
161]. These conditions suggest the presence of an underlying inflammatory pathway may be involved, impacting various organ systems and intensifying the overall symptom burden [
162,
163,
164]. Continuous activation of the immune system in these conditions perpetuates the inflammatory state, therefore underscoring the complex nature of fibromyalgia [
160,
161].
In conclusion, the interplay between peripheral inflammation and fibromyalgia underscores the importance of inflammation as a key factor in the onset and progression of the disease. Inflammatory cytokines and activation of the immune cells in the periphery can directly affect the function of the nervous system, enhancing excitability of pain pathways and leading to an exaggerated pain response. Consequently, a self-sustaining feedback loop is established, where pain, inflammation, and immune dysfunction reinforce each other. However, these interconnections complicate the understanding of the underlying mechanisms.
3.2. Central Inflammation and Fibromyalgia
Fibromyalgia has been increasingly recognized as a disorder intricately connected to central inflammation, with neuroinflammation emerging as an essential component of its pathophysiology [
5,
165,
166]. Central to this process is the activation of glial cells, particularly microglia and astrocytes. Upon activation, these cells initiate a series of inflammatory events, resulting in the release of many pro-inflammatory cytokines, including IL-1β, IL-6, IL-8, IL-10, TNF-α, BDNF, and GDNF, among others [
167,
168]. Several investigations have shown elevated levels of some of the aforementioned cytokines in the CSF of fibromyalgia patients (
Table 2), indicating a pervasive state of central inflammation [
136,
137,
138,
169]. Moreover, recent studies have emphasized the significance of S100 proteins in the field of fibromyalgia research [
170]. These proteins are involved in many inflammatory processes, and they may exert a considerable influence on fibromyalgia’s development and progression. The role of S100 proteins in fibromyalgia may be mediated through RAGE and TLR4, which, in turn, activate signaling pathways that promote the release of several pro-inflammatory cytokines (
Figure 2), as previously mentioned [
170].
Central sensitization enhances pain signaling, converting otherwise innocuous stimuli into significant sources of discomfort and contributing to the chronic and widespread pain characteristic of fibromyalgia. In addition to pain amplification, dysregulation of inflammatory processes leads to an imbalance between pro-inflammatory and anti-inflammatory cytokines [
171]. As a result, the neuroinflammatory state is sustained, establishing a feedback loop that further worsens the condition [
8]. Neuroimaging studies have further corroborated these findings, revealing microglial activation in patients with fibromyalgia [
8,
11]. The consequences of this persistent neuroinflammatory state extend beyond pain hypersensitivity, playing a key role in the development of a broad spectrum of debilitating symptoms commonly associated with fibromyalgia [
172]. Cognitive dysfunction, often referred to as “fibro fog”, serves as an additional manifestation of this inflammatory state, likely stemming from the detrimental effects of cytokines on neural connectivity and synaptic function [
173]. Sleep disturbances, which are both a symptom and a contributing factor to fibromyalgia, may be intricately linked to the inflammatory process, as cytokines have the ability to affect sleep regulation and disrupt restorative sleep cycles [
174,
175].
On the other hand, obesity exerts a profound and multifaceted influence on central inflammation in fibromyalgia patients [
176,
177]. The relationship between obesity and fibromyalgia is complex and bidirectional, with obesity potentially serving as a risk factor and an aggravating factor for this condition [
178]. Obesity is involved in central inflammation through some mechanisms, primarily the secretion of pro-inflammatory cytokines and chemokines such as TNF-α, IL-6, CCL4, and CCL13 by adipose tissue, primarily due to macrophages [
179,
180,
181,
182]. These cytokines, in addition to exerting their effects on the sensitization of nociceptors and dorsal root ganglia (DRGs) [
183], can cross the blood–brain barrier (BBB) and have the potential to activate microglial cells placed in the CNS, thereby maintaining a state of neuroinflammation (
Figure 3) [
184,
185]. Moreover, a recent study has shown that obesity in fibromyalgia patients acts as a disruptor of the descending pain pathway, thereby exacerbating symptoms associated with this condition [
186].
Obesity is closely linked to metabolic dysregulation, including insulin resistance and leptin resistance, both of which play a role in the modulation of pain perception and neuroinflammation [
187]. Higher levels of leptin have been found to correlate with increased pain sensitivity and inflammatory markers in fibromyalgia patients, suggesting a direct relationship between adiposity and central pain processing [
188,
189]. Furthermore, obesity exacerbates systemic low-grade inflammation, which amplifies central sensitization processes characteristic of fibromyalgia, establishing a vicious cycle in which pain and inflammation sustain each other [
190]. The comorbidity of obesity and fibromyalgia is also linked to worsened clinical outcomes (such as higher pain intensity) and reduced physical function; however, increased fatigue can further compromise quality of life [
191]. In addition to these effects, obesity usually results in sleep disturbances and obstructive sleep apnea, both of which are prevalent in fibromyalgia and contribute to the further disruption of inflammatory and neuroendocrine pathways [
176].
Common behavioral and lifestyle factors, including physical inactivity and poor dietary habits, also contribute to both conditions, perpetuating the inflammatory state and hindering effective management [
190]. Given this interplay, addressing obesity in fibromyalgia patients through weight management, anti-inflammatory interventions, and lifestyle modifications may offer a promising therapeutic approach to mitigate central inflammation, reduce symptom severity, and improve overall health outcomes. Nevertheless, it is very important to recognize the complexity of these interactions, as they have a significant impact on treatment effectiveness. Although these factors are crucial, additional underlying mechanisms must also be explored to obtain a thorough understanding of their impact on patient health.