1. Introduction
The field of microfluidics offers great potential to miniaturize processes in chemistry, biochemistry, and biology. Miniaturization of reactions and analytical processes can lead to a reduction in reaction time and reactant’s volume and facilitate parallelization [
1,
2]. The processing time can be reduced as mixing and heating occur faster within smaller volumes [
3]. Furthermore, it is advantageous for analytical applications to run the experiment within a smaller sample size. This especially holds true for biological or medical analytics, where the samples are difficult to acquire or the sample volume is very small. Furthermore, for chemical applications, hazardous reactions can be scaled down in order to reduce the risk of critical incidents [
4,
5].
Over the past few decades, additive manufacturing (AM) has been proven as a convenient tool for microfluidics fabrication due to its low cost [
6], arbitrary design [
7], and one-step fabrication [
8]. Assisted by computer-aided design (CAD), the 3D printing process allows for facile alternation and adaptation of prospective geometries at various stages of the development process, decreasing the concept-to-chip time [
9]. Different technologies of AM have been successfully used for microfluidic fabrication such as digital light processing (DLP) [
10,
11], laser-based stereolithography (SL) [
12], two-photon lithography [
13,
14], inkjet printing [
15], suspended liquid subtractive lithography (SLSL) [
16], and fused deposition modeling (FDM) [
17,
18,
19]. The scope of microfluidic devices fabricated by AM is expanded continuously, and AM-fabricated microfluidics have been used in cell culturing to create microgels for cell encapsulation [
20] and encapsulations for stem cell engineering [
21,
22]. Three-dimensionally printed integrated microfluidics have been utilized for flow rate sensing [
23] and amperometric detection in electrophoresis [
24]. Furthermore, on-chip chemical synthesis has been shown to synthesize PET tracers [
25], nucleoside analogs [
26], and self-heating microreactors have been demonstrated [
27]. FDM printing stands out as one of the most widespread AM technologies due to its simplicity and low cost of investment and operation. A wide variety of thermoplastic materials can be processed with FDM, such as polylactic acid (PLA), acrylonitrile butadiene styrene (ABS), poly(ethylene terephthalate) (PET), and polycarbonate (PC). Recently, our lab also demonstrated FDM fabrication of microfluidic chips in poly(methyl methacrylate) (PMMA) and polystyrene (PS) [
17,
18]. These materials are used for large-scale production methods like hot embossing or injection molding. This makes FDM printing a preferable AM method for prototyping, as the transfer from lab-scale to industrial processes is easier to conduct [
28].
Despite their industrial abundance and good optical properties, most FDM materials suffer from low chemical resistance, especially against organic solvents [
29,
30]. Furthermore, most of these materials have low heat resistance, and their Vicat softening points are commonly located well below 100 °C [
31]. This limits the versatility of the devices manufactured with FDM, as they prevent the application of microfluidic chips in environments that call for high-performance material properties. A potential material of choice for this could be fused silica, which has outstanding chemical and heat resistance. FDM printing of silica by using a thermoplastic nanocomposite, which is subsequently debinded and sintered, has been demonstrated [
32]. Nevertheless, the process is challenging and requires a multi-step protocol in order to generate functional chips. Therefore, there is a demand for material that can be processed with FDM and can yield microfluidic chips with improved thermal and chemical stability compared to conventional FDM polymers.
Fluorinated polymers are superior regarding their chemical resistance, good mechanical strength, and UV stability. Additionally, they mostly exhibit high melting points [
33]. The reason for their low reactivity and swelling in organic solvents is the short proximity within the carbon–fluorine bond [
34]. The distance between both atoms is 1.35 Å, making it the shortest of all covalent bonds in organic molecules and the reason why intermolecular interactions with the carbon atoms of the polymer backbone are reduced in comparison to non-fluorinated polymers [
35]. Additional to the chemical resistance, their thermal stability can be advantageous for microfluidic reactions that require high temperatures to accelerate reaction speed. For example, a typical polymerase chain reaction protocol involves heating to >90 °C, which exceeds the working temperature of PLA [
36]. In comparison, fluorinated thermoplasts are well suited for these temperatures due to their typically high melting points of common commercial fluorinated thermoplasts such as fluorinated ethylene propylene (FEP, m
p: 260 °C) or polytetrafluoroethylene (PTFE, m
p: 327 °C) [
37]. Nevertheless, these temperatures are not suitable for most FDM printers. Polyvinylidene fluoride (PVDF, m
p: 177 °C) is a thermoplastic polymer that exhibits the advantages of fluorinated polymers and has a melting point that is low enough to be processed with generic FDM printers [
38]. The surface energy of the difluoromethylene group (-CF
2-) is one of the lowest found in organic molecules at ~18 mN/m [
39]. Its maximum continuous service temperature is 150 °C and therefore higher than most conventional FDM materials [
31]. These outstanding properties make fluorinated polymers the ideal material for microfluidic chemical reactors.
In this work, we demonstrate for the first time FDM printing of microfluidic chips in PVDF in order to fabricate high-performance microfluidics in a simple one-step protocol. We show how to eliminate warping of the material during printing and to increase transparency by printing directly on a glass substrate. Furthermore, we demonstrate the high chemical resistance of the functional microfluidic chips made of PVDF in applications that would be unfeasible with conventional FDM materials. Applying our FDM protocol to prototype microfluidics with high-performance properties will open new possibilities for lab-on-a-chip (LoC) applications in the field of chemical synthesis.
2. Materials and Methods
2.1. Materials
The filament 3DXTech FluorX PVDF with a diameter of 2.85 mm was purchased from Filamentworld (Neu-Ulm, Germany). Acetone, acetone-d6, 2-propanol, methanol (MeOH), dichloromethane (DCM), dimethylformamide (DMF), tetrahydrofuran (THF), toluene, n-heptane, diphenyl(2,4,6-trimethylbenzoyl) phosphine oxide (TPO), and strongly acidic ion exchanger resin Amberlyst 15 (hydrogen form, dry) were purchased from Merck (Darmstadt, Germany). Bromothymol blue, phenolphthalein, and toluene sulfonic acid monohydrate were purchased from Carl Roth GmbH & Co., KG (Karlsruhe, Germany). Fluorolink MD700 (MD700) was obtained from Acota (Oswestry, UK). Elastosil RT 601 A/B was purchased from Wacker (Munich, Germany). Benzaldehyde dimethyl acetal was purchased from BLD Pharm (Shanghai, China).
2.2. Fused Deposition Modeling
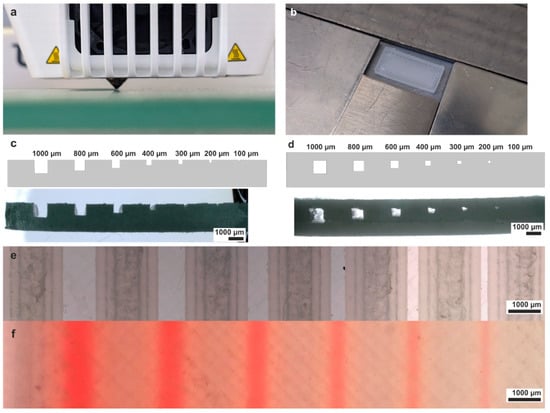
The CAD models for the FDM printing were designed using Autodesk Inventor Professional 2023 (Autodesk, San Rafael, CA, USA) and exported as STL files. The STL files were sliced using the slicing software Ultimaker Cura 4.7.1. (Ultimaker B.V., Utrecht, The Netherlands). Three-dimensional printing was conducted on a commercial 3D printer (Ultimaker 3, Ultimaker B.V., Utrecht, The Netherlands). A nozzle diameter of 0.4 mm was chosen. The printing temperature of the initial layer was 190 °C and the printing temperature of the residual layers was 230 °C. The built plate temperature was set to 95 °C for all layers. The printing parameters were optimized for the fabrication of microfluidic devices. The layer thickness was set to 100 µm and the line width to 350 µm. The printing and infill speeds were set to 30 mm/s, and the infill density and wall infill flowrate were set to 100% and 87%, respectively. To ensure the adhesion of the prints to the printing bed, adhesion spray 3DLac (niceshops GmbH, Germany) was applied to the platform, and a brim of 7 mm width was added while printing the first layer.
For evaluation of the channel resolution, a series of embedded square-shaped channels with different diameters were printed, cut with a precision saw, Diadisc 5200 (Mutronic, Rieden am Forggensee, Germany), and then polished with a diamond mill. The cross-section was analyzed by a light microscope of type VHX6000 (Keyence, Osaka, Japan).
2.3. Casting of PDMS and PFPE Samples
Two reference materials were cast to compare the swelling in common solvents with the 3D-printed PVDF. Polydimethylsiloxane (PDMS) samples were cast by mixing Elastosil RT 601 A/B in a ratio of 9:1 (A to B, by weight) and poured into 200 µL cavities. Prior to casting, entrapped air bubbles were removed using a vacuum desiccator. The PDMS was cured at 65 °C for 2 h, then allowed to cool to room temperature and carefully removed from the mold. For the second benchmark substance, the photoinitiator TPO (1 wt%) was dissolved in the highly fluorinated perfluoropolyether (PFPE) methacrylate MD700 at room temperature. The photoresist was cast into 200 µL cavities and cured at 365 nm for 10 min. After curing, the photo-crosslinked PFPE acrylates were carefully removed from the mold. A comparable mixture has been utilized by our group for 3D printing of microfluidic chips via vat-photopolymerization [
39].
2.4. Solvent Compatibility
Solvent compatibility of the printed PVDF material was determined using 10 mm × 10 mm × 3 mm printed blocks of PVDF. The weight of each block was determined three times, and the block was immersed in 20 mL of the respective solvent (water, MeOH, DCM, DMF, THF, toluene, acetone, and n-heptane) for 24 h. After immersion, the blocks were cleaned with a tissue, and the weight after swelling was determined immediately. The block was then re-immersed in the same solvent and, after weighting, the next eight blocks. A total of three measurements were taken for each of the three blocks.
2.5. White Light Interferometry and Roughness Determination
The surface roughness of 3D-printed samples was analyzed by white light interferometry (WLI) (NewView 9000, Zygo, Middlefield, CT, USA). The surface roughness measurements were carried out on an area of 1.6 × 1.6 mm2 at three different positions, calculating the surface roughness Sq and mean line roughness Ra with Gwyddion.
2.6. Scanning Electron Microscopy
Scanning electron microscopy (SEM) was carried out on a Quanta 250 FEG (FEI Inc., Valley City, ND, USA) with an accelerating potential of 5 kV. Samples were prepared with conductive tape on SEM sample holders and conductive silver paint and were sputtered with a gold–palladium layer of about 25 nm thickness prior to SEM imaging.
2.7. Microfluidic Experiments
To investigate the functionality of the FDM-printed microfluidic chips, the channels were filled with fluids using a syringe pump (Legato 210, KDScientific, Holliston, MA, USA). To connect the syringe with the chips, PTFE tubes were plugged onto a 3D-printed chip-to-world interface and fixed with epoxy glue. Pumping rates up to 10 mL/min were used to perfuse the microfluidic chips.
2.8. 1H-NMR Spectroscopy
The 1H-NMR spectra were measured on a Bruker Avance III HD 300 MHz NMR spectrometer. The solvent used was aceton-d6, which was also used as the reaction solvent. All signals were referenced to an internal solvent signal (2.05 ppm). The evaluation of NMR spectra was performed using the software MestreNova 15.0 (Mestrelab Research, Santiago de Compostela, Spain).